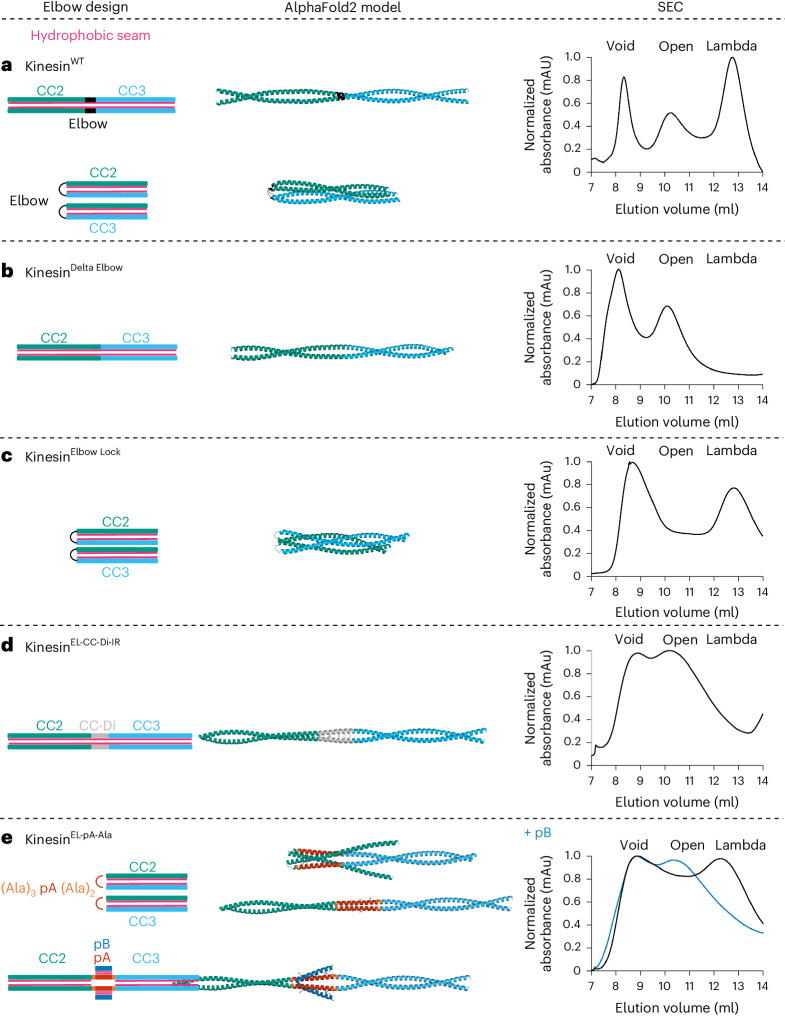
Fig. 2. Engineering a conformational switch into kinesin-1 for allosteric activation by a de novo designed peptide.
From left to right: cartoon illustrations of each elbow design; AlphaFold2 models of KHC for the CC2-linker-CC3 region; and SEC elution profiles of purified heterotetrameric KHC–KLC complexes without (black) and with (blue) peptide pB. Color scheme for the structural cartoons: KHC CC2 teal and CC3 cyan; hydrophobic seams/cores, pink; CC-Di, gray; peptide pA, red; flanks, yellow; peptide pB, blue. a, Wild-type kinesin-1 (Kinesin-1WT) exists in an equilibrium between the open and lambda states. b, The delta elbow variant of kinesin-1 (Kinesin-1Delta Elbow), with an 18-residue deletion in the elbow region, favors the open state. c, The elbow lock variant (Kinesin-1Elbow Lock), which has a five-residue deletion in the elbow region, favors the lambda state. d, Introduction of a 28-residue de novo designed peptide CC-Di in place of the deleted five-residue elbow of kinesin-1 (Kinesin-1EL-CC-Di-IR) favors the open state. e, Designed switchable variant KinesinEL-pA-Ala without (top) and with (bottom) peptide pB. Insertion of pA with a shifted coiled-coil register relative to CC2 and CC3 using helix-favoring (Ala)3 and (Ala)2 flanks, respectively, allows flexibility in the elbow without pB favoring the lambda particle, but induced coiled-coil formation with pB activates the switch to the open state. AlphaFold models predict both an open and alternative compact state without peptide pB, but all open models with pB.