Abstract
Autophagy and mitophagy pose unresolved challenges in understanding the pathology of diabetic heart condition (DHC), which encompasses a complex range of cardiovascular issues linked to diabetes and associated cardiomyopathies. Despite significant progress in reducing mortality rates from cardiovascular diseases (CVDs), heart failure remains a major cause of increased morbidity among diabetic patients. These cellular processes are essential for maintaining cellular balance and removing damaged or dysfunctional components, and their involvement in the development of diabetic heart disease makes them attractive targets for diagnosis and treatment. While a variety of conventional diagnostic and therapeutic strategies are available, DHC continues to present a significant challenge. Point-of-care diagnostics, supported by nanobiosensing techniques, offer a promising alternative for these complex scenarios. Although conventional medications have been widely used in DHC patients, they raise several concerns regarding various physiological aspects. Modern medicine places great emphasis on the application of nanotechnology to target autophagy and mitophagy in DHC, offering a promising approach to deliver drugs beyond the limitations of traditional therapies. This article aims to explore the potential connections between autophagy, mitophagy and DHC, while also discussing the promise of nanotechnology-based theranostic interventions that specifically target these molecular pathways.
Keywords: Autophagy, Diabetes, Diabetic heart condition, Mitophagy, Nanotheranostics, Nanomedicine
Graphical abstract
1. Introduction
Diabetes is a chronic metabolic syndrome that significantly increases the risk of cardiac disorders, with the treatment of cardiovascular diseases (CVDs) accounting for the majority of diabetes-related healthcare costs [1,2]. DHC is a complex array of cardiovascular ailments and subsequent myopathies resulting from chronic diabetes. Clinical evidence has consistently linked diabetes to an elevated risk of heart failure and cardiac diseases. Compared to non-diabetic individuals, diabetes patients face a more than twofold higher continuous threat of CVDs [3], with heart failure being among the most severe in terms of prognosis [4]. The association between diabetes and heart failure primarily involves ischemia and metabolic abnormalities, such as hyperglycemia-induced gluco‑toxicity and insulin resistance-mediated lipo-toxicity [5]. Proper diagnosis, coupled with a comprehensive understanding of the disease pathogenesis and advanced treatment of diabetes-related cardiac disease, is crucial to reduce mortality rates among affected individuals [1].
Dysfunctional autophagy is one of the root causes of diabetes and age-related cardiac issues. Autophagy is a highly conserved process involving the intracellular degradation of waste products in the cytoplasm by lysosomes [6]. Emerging evidence has linked autophagy impairments to various cardiovascular complications, including diabetic cardiomyopathy (DCM). Mitophagy, a specialized form of autophagy dedicated to the degradation and elimination of mitochondria, is crucial for maintaining mitochondrial integrity and ensuring cell viability [7,8]. Since both autophagy and mitophagy have dual functions of dysregulation and protective contributions to maintaining cardiac homeostasis, balancing them is crucial to maximize protective functions and minimize dysregulation in CVD [9]. Variations in the levels of autophagy and mitophagy may contribute to the progression of DHC [3,10], making them promising diagnostic and therapeutic biomarkers. Despite diabetic patients often experiencing cardiac issues, CVD and DCM are frequently undiagnosed or poorly identified. The prevalence of diabetic CVD can be reduced with effective diabetes treatment from the early stages of the disease, but this is often challenging due to various factors [11]. In many cases, diabetes and its associated cardiac diseases remain asymptomatic, making diagnosis and treatment even more challenging. Developing strategies for efficient point-of-care diagnosis and timely treatment can significantly reduce the morbidity associated with CVDs. Traditional diagnostic and therapeutic interventions can be improved with the help of nanotechnology to provide an enhanced approach to managing cardiac complications [12,13].
Nanotechnology involves the formulation, manipulation, or engineering of materials, structures, devices, and particles to impart novel properties due to their nanoscale dimensions [14]. It bridges the gap between biology and medicine for various therapeutic purposes. Since Richard Feynman introduced the concept of nanotechnology, it has gained immense importance in fields such as agriculture, food, cosmetics, and particularly medicine. One of the primary applications of nanotechnology is nanotheranostics, where medicinal tools and nanotechnological interventions are used to efficiently diagnose and treat diseases [14,15].
Nano-biosensors have been designed to generate signals corresponding to the concentration of diagnostic biomarkers [16]. Nanocomposites are being used in biosensor production to enhance their sensitivity and functionality. Numerous innovative technologies for analyzing the autophagy and mitophagy pathways have been incorporated into biosensors due to the use of these nanomaterials. With the combination of chemistry, health sciences, and nanotechnology, biosensors can quickly assess the health status. Conventional therapeutic interventions available in the market have limitations such as less target specificity, modified impacts, and reduced effectiveness due to drug metabolism, leading to the development of nanomedicines for DHC treatment [17,18]. Nanomedicine involves treating and preventing diseases, aiming to improve individuals' lifestyles [19]. The use of nanotechnologies in medicine, particularly in developing novel drug-delivery techniques for DHC and associated disorders, has become increasingly prevalent [20]. Nanoscale carriers can target affected areas specifically, enhancing treatment effectiveness and reducing unwanted adverse effects. Surface modification and stimuli-responsive functionalization can further enhance the targeting potential and therapeutic efficiency of nanosystem-assisted drug delivery [21]. This article aims to provide a pioneer optimistic insight into the contributory roles of autophagy and mitophagy in DHC and nanotechnology-mediated theranostic interventions for preventing cardiac and metabolic ailments associated with DHC.
2. Role of autophagy in diabetic heart condition
Autophagy is a fundamental process that maintains overall homeostasis and stability in an organism under standard physiological conditions. It becomes crucial for efficiently transporting and targeting cellular debris, abnormal protein conformations, and other toxic components to the lysosome for effective degradation. This degradative recycling pathway helps cells survive in various unfavorable conditions. Autophagy occurs in two steps: first, double-membrane autophagosomes are formed, which engulf internal cargos, and then they merge with lysosomes to initiate degradation with the help of lysosomal enzymes. In cardiac cells, persistent hyperglycemia may hinder autophagy [22]. Dysfunctional autophagy has been linked to malfunctions and irregularities in diabetic hearts. As a critical physiological process, autophagy plays a regulatory role in several pathological processes associated with DHC, encompassing various CVDs, DCM and multiple aspects of diabetes [23].
DCM affects heart tissue and compromises heart function, leading to mortality in diabetic individuals. Imbalanced lipids in diabetes have been suggested to be induced by dysfunctional autophagy caused by the inhibition of adenosine monophosphate (AMP)-activated protein kinase (AMPK), which may contribute to DCM [24]. AMPK acts as an energy sensor critical for maintaining cellular energy balance. When cellular energy is low, indicated by a high intracellular AMP/ATP (adenosine triphosphate) ratio, AMPK is activated. This stimulates metabolic processes that generate ATP and downregulates pathways that use ATP [25]. Functional AMPK can also inhibit cell growth and lipid production while promoting glucose absorption and autophagy. Insulin trafficking promotes the activation of the mammalian target of rapamycin (mTOR), which inhibits autophagy and autophagy-related 1 protein (Atg1) [26,27]. Specifically, activation of mammalian unc-51-like kinase 1 (ULK1) by AMPK and mTOR complex 1 (mTORC1) initiates autophagy [28]. AMPK can limit mTORC1 function, accelerate the degradation of unnecessary cellular components, and improve ATP production [29,30]. The heart, which is normally hypermetabolic and consumes significant oxygen, is regulated by Forkhead box protein O1 (FOXO1), a metabolic and antioxidant regulator. FOXO1 plays crucial roles, particularly during DCM, characterized by metabolic abnormalities. The predominant isoform, FOXO3, present in the heart, is crucial in several cardiac disorders. FOXO1/3, a known regulator of catabolic processes, is essential for autophagy. They bind to the regulatory regions of autophagy-related genes (Atgs) and activate cardiomyocyte autophagy by increasing the expression of these genes [31]. Activation of FOXO1/3 enhances cardiomyocyte autophagy and provides additional protection against chronic medical conditions [32].
In insulin-dependent diabetes, impaired insulin trafficking leads to the stimulation of cardiac autophagy [33]. Gluttony and adiposity in adult-onset diabetes inhibit the breakdown of damaged cells, leading to elevated intracellular levels of precursors such as glucose and/or free fatty acids. This dysregulation of insulin transmission boosts autophagy. Autophagy has been implicated in the development of type 2 diabetes (T2D) through mechanisms affecting damaged pancreatic beta cells and the emergence of insulin resistance [34]. Studies have shown that rodents deficient in autophagy-related 7 protein have fewer pancreatic beta cells, reduced glucose tolerance, and decreased insulin secretion. In insulin-resistant rodent models, autophagy is essential for preventing diabetes and maintaining the structure and function of pancreatic beta cells [35]. However, highly activated autophagy can harm pancreatic beta cells, leading to their apoptosis. The marginalization of autophagy in cardiovascular tissues in T2D is caused by reduced sirtuin 1 activity, which, when activated, reduces oxidative stress, inflammation, and cell death while promoting autophagy and AMPK activation [36]. Reduced AMPK activity in T2D often coincides with decreased cardiac autophagy [37].
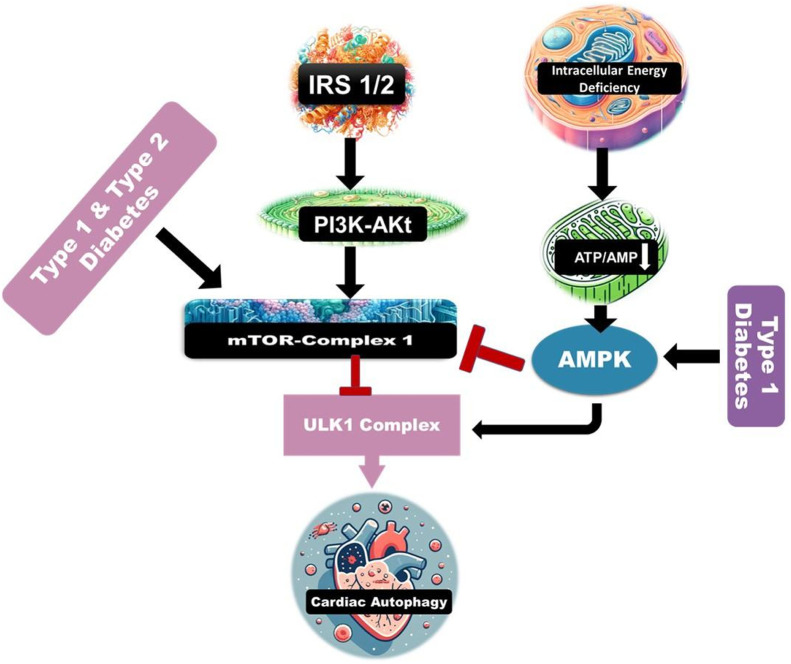
Dysregulation of AMPK signaling suppresses autophagy and is a key factor in DCM, as cardiac-specific mutations occur in SIRT1 in T2D (Fig. 1). In a rodent model of DCM, activating AMPK with resveratrol boosts autophagy via the mTOR pathway, enhancing cardiac performance without affecting blood glucose levels [38]. Patients with T2D experience cardioprotection against oxidative stress in pancreatic beta cells due to increased autophagy. However, cardiac dysfunction in T2D can occur due to suppressed lysosomal activity caused by impaired autophagosome clearance. While rapid autophagy activation may help the heart compensate for energy deficiency, sustained elevation of autophagy could be harmful due to a lack of substrate for degradation. Therefore, it will be crucial to employ efficient methods and optimize the duration and timing of interventions if autophagy is to be used as a therapeutic target [39]. It is predicted that both juvenile and adult-onset diabetes can enhance autophagy efficiency.
Fig. 1.
Important molecular signals controlling autophagy in DHC. Cardiac autophagy is inhibited by diabetes. AMPK is inhibited, and mTOR is activated in T1D and T2D, which then deactivates ULK1, a kinase required to begin autophagy. The autophagy process is initiated following mTOR inhibition or activation of the AMPK signalling pathway.
Due to physiological conditions like nutrient deprivation, autophagy is firmly triggered in the heart, within the secluded cardiomyocytes. Under those circumstances, autophagy is crucial for the degradation of protein aggregates and the retention of organelles at low basal levels. Autophagy is also activated under conditions of cardiovascular stress, such as ischemia and heart failure, cardiac hypertrophy, and cardiomyopathies [40].
Autophagy can also be seen in ischemia, where blood flow is impeded or decreased in a particular body part. Reduced oxygen and nutrient availability lower cytosolic ATP synthesis, activating AMPK and inhibiting mTOR, both of which promote autophagy to maintain cellular levels of energy and cardiomyocyte feasibility [41]. Autophagy is initiated by the concurrent introduction of an effective mTOR antagonist, 3HOI-BA-01 ((E)-3-(4-(benzo[d][1,3]dioxol-5-yl)-2-oxobut-3-en-1-yl)-3-hydroxyindolin-2-one) and a precise inducer of AMPK, PT1. It declines the death of cardiomyocytes activated by modeled ischemia [42]. As a result, it has been demonstrated that a wide range of autophagy inducers, such as trehalose and spermidine, can reduce myocardial damage and enhance the cardiac consequences of ischemic injury in rodents by stimulation of autophagy [43]. It is observed that right atrium attachments obtained from human T2D patients with myocardial ischemia having coronary artery bypass transplantation have higher numbers of light chain 3 beta-positive muscle cells, light chain 3 beta II protein expression, and autophagosomes. Evidently, the heart of the diabetic person has autophagy turned on. Autophagy also serves as a regulatory mechanism in cardiac hypertrophy to preserve the standard framework and operation of the heart. This is proved by the cardiac-specific deletion of autophagy-related 5 (Atg5) that leads to hypertrophic cardiomyopathy, impairment in the contraction of heart muscles and increased vulnerability to pressure overload. Reactive oxygen species (ROS) harm cardiomyocytes, but autophagy reverses this damage by expelling deteriorated mitochondria from hypertrophic hearts [44]. By targeting ubiquilin-1 (UBQLN1), an important aspect of the mTOR pathway, miR-377-5p silencing reduces cardiac hypertrophy [45]. miR-34a reduced cardiomyocyte hypertrophy in a rat model, which is introduced with Ang II by silencing the Atg9A expression, a protein that is associated with the formation of autophagosomes. As a result, suppressive impact of miR-34a on cardiac hypertrophy by attenuating Atg9A expression implied that Atg9A may be a priority attraction for miR-34a-targeted therapies for cardiac hypertrophy [46]. Also, in the absence of myeloid leukaemia 1, a member of the B cell leukaemia 2 family that usually inhibits apoptosis, also disrupts stress-activated autophagy, making it difficult for the heart to pump the blood to the rest of the body and induce apoptosis [47]. Heart failure (HF) follows cardiac hypertrophy if there is continuous stress because it is the pretty standard end point of all cardiac diseases. HF with reduced or preserved ejection fraction affects a similar percentage of patients. Research findings on heart failure with reduced ejection fraction (HFrEF) caused by pressure overload indicated that cardiomyocyte homeostasis and hemodynamic stress in the heart are controlled by autophagy [48]. According to a study, pressure overload temporarily activates autophagy, which subsequently reduces, promoting the growth of HFrEF. To favor this study, revitalizing autophagy by TAT-Beclin 1 peptide under pressure overload reduces HFrEF [49]. Uncontrolled autophagy can aggravate HF disorder. For instance, the knockout of lymphocyte antigen 86 (Ly86) leads to the advancement of mice bearing HF with preserved ejection fraction (HFpEF) by increasing autophagy through inducing the reactive oxygen species–mitogen-activated protein kinases (ROS-MAPK) signaling cascade. Furthermore, through the modification of mTOR complex 2 and its signaling, activation of AMPK enervates uncontrolled autophagy and hinders HF [50,51]. These studies show that autophagy is essential for healthy heart function.
3. Role of mitophagy in diabetic heart condition
Mitophagy is a subtype of selective autophagy, which is responsible for the specific targeting and degradation of damaged or dysfunctional mitochondria. Unsurprisingly, the mitophagy and autophagy pathways share extensive similarities [52,53]. In the canonical pathway of mitophagy, a group of proteins that are associated with mitophagy, such as Atg7 and Atg5, are crucial to the formation of the autophagosome. In mammalian cells, mitophagy is mainly facilitated through the PINK1/Parkin pathway, which involves the coordinated action of the proteins PINK1 (PTEN Induced Kinase 1) and Parkin to identify and tag damaged mitochondria for degradation [54]. Under diabetic conditions, hyperglycemia and relatively higher free fatty acid concentration induce damage to mitochondria, which often leads to the loss of mitochondrial membrane potential (ΔΨm). This is a well-known and common trigger of mitophagy [55,56]. In such cellular stress conditions, the import of full-length PINK1 into mitochondria via the translocase of the outer mitochondrial membrane (TOM) and inner mitochondrial membrane (TIM) complexes is impaired. As a result, PINK1 accumulates on the outer mitochondrial membrane (OMM), where it stabilizes on the OMM in a complex comprising of TOM7, TOM20, TOM22, TOM40, and TOM70 proteins [57] and gets activated [58]. After PINK1 gets activated, it phosphorylates specific mitochondrial proteins, including mitofusin 2 (Mfn2), which acts as a receptor to recruit Parkin [59], an E3 ubiquitin ligase protein.
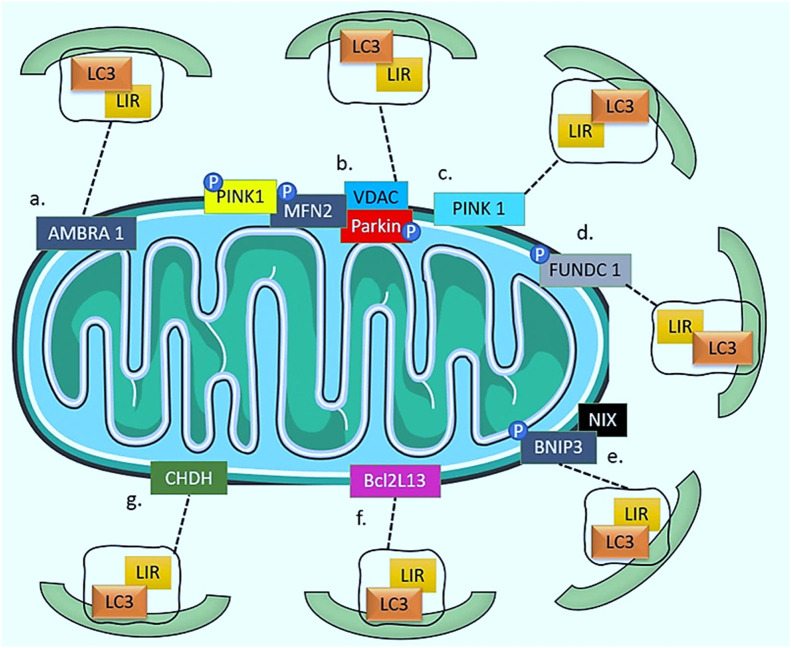
PINK1 has also been found to be recruited by various pathways (Fig. 2). The most prominent among them is the Parkin/PINK1 pathway. In this pathway, the initiation of the formation of autophagosome is driven by the activation of Unc51-like kinase 1 (ULK1) and Unc51-like kinase 2 (ULK2) complexes. mTOR has been reported to inhibit this activation process. This step is followed by nucleation step. The nucleation process is dependent on the Beclin 1-Vps34-Vps15 core complexes and other associated proteins. Ultimately, the elongation of autophagosome is mediated by the autophagy-related (ATG) proteins ATG12 and ATG5, which participate in the elongation of the isolation membrane [[60], [61], [62]]. Apart from this, it has been widely seen that PINK1 can also be recruited by the AMBRA1 (Autophagy And Beclin 1 Regulator 1), FUN14 domain containing 1 (FUNDC1), NIX/BNIP3, BCL2L13 (B Cell Leukemia 2-Like 13) dependent LCR-LIR (LC3-interacting region) binding, and Choline dehydrogenase (CHDH), and/or by LC3-independent manner [63].
Fig. 2.
Diagrammatic representation of Parkin-dependent and independent pathways: (a) AMBRA1 pathway, (b and c) Parkin/PINK1 pathway, (d) FUNDC1 pathway, (e) NIX or Bnip3 pathway, (f) BCL2L13 pathway, (g) CHDH pathway.
The binding of AMBRA1 to LC3 results in the anchoring of damaged mitochondria to the autophagosomal membrane. Following this event, activation of class III PI3K by AMBRA1 leads to the formation of a mitophagosome. However, despite the ability of Parkin to enhance the recruitment of Ambra1 to mitochondria and the role of p62 in mitochondrial perinuclear clustering, this process is not dependent on either Parkin or p62 [64]. It should be noted that while Ambra1 is a mitophagy receptor that interacts with LC3 through its LIR, its presence on the OMM does not eliminate the need for other mitophagic receptors to identify and label damaged or dysfunctional mitochondria. Rather, AMBRA1 may work in concert with other mitophagic receptors to facilitate the efficient and selective removal of damaged mitochondria through their direct interaction with LC3. Therefore, the presence of multiple mitophagic receptors with distinct properties and functions is necessary for the proper regulation of the mitophagy process [65]. The mitophagy receptor, FUNDC1 protein, is present on the OMM that recruits autophagosomes via its LC3-binding motif, which is analogous to that of NIX/BNIP3. FUNDC1-induced mitophagy requires Atg5 but not Beclin 1 and does not need Parkin either [66,67]. The coordination of mitochondrial fission, fusion, and mitophagy to maintain the quality control of mitochondria has been shown to be aided by FUNDC1′s interaction with dynamin-related protein 1 (DRP1) and Optic Atrophy 1 (OPA1) [68,69]. The protein BNIP3, a member of the Bcl-2 family, has a role in promoting cell death. NIX, a homolog of BNIP3, a transmembrane protein found on the OMM [70]. BNIP3 and NIX modulate mitophagy directly by interacting with LC3II and gamma-aminobutyric acid receptor-associated protein (GABARAP), respectively, via their N-terminal LC3-interacting motifs [71]. Moreover, mitochondrial depolarization, which has been shown to be capable of triggering mitophagy on its own, may be induced by [72]. BCL2L13 is localized on the OMM and establishes an interaction with LC3 via its WXXI motif, which is necessary for LC3-mediated mitophagy. Unlike the typical process involving Parkin, BCL2L13 triggers mitophagy without requiring mitochondrial ubiquitination [73]. CHDH converts choline into betaine aldehyde in the mitochondria. Under normal conditions, CHDH is not anchored to either the OMM or IMM. The FAD/NAD-binding domain 1 of CHDH interacts with the Phox and Bem1 domains of the cytoplasmic p62/SQSTM1, recruiting it to the depolarized mitochondria and facilitating it to attach with LC3 [74]. Additionally, the monophosphate-activated protein kinase (AMPK) can induce mitophagy in a Parkin-independent manner through phosphorylation and activation of TANK-Binding Kinase 1 (TBK1) [75].
The process of mitophagy can be facilitated by the formation of Rab9-mediated autophagosomes. These autophagosomes are formed through the interaction between the protein Rab9, ULK1, and DRP1 protein [76]. The mitophagy protein BECN1/Beclin1 has a vital contribution to the formation and maturation of autophagosomes, and it also interacts with Parkin. However, its function is independent of Parkinʼs translocation to the mitochondria [77].
The presence of these poly-ubiquitinated chains on the surface of the mitochondria results in the recruitment of both the ubiquitin-proteasome system (UPS) machinery and the mitophagy machinery [78]. This induces the recruitment of specific Ub-binding mitophagy receptors through the ubiquitin-binding domain. Following this event, P62 or optineurin (OPTN) recruits autophagosomal membranes via LC3 through their LC3-interacting domain. This leads to the formation of mitophagosomes. These mitophagosomes eventually get fused with lysosomes where enzymatic degradation of mitochondria takes place [79]. Investigating this pathway is highly crucial for a detailed insight on the role of mitophagy in DHC. The molecular components can act as a potential therapeutic target of the latest biomedical nanoparticles and nano-conjugated systems.
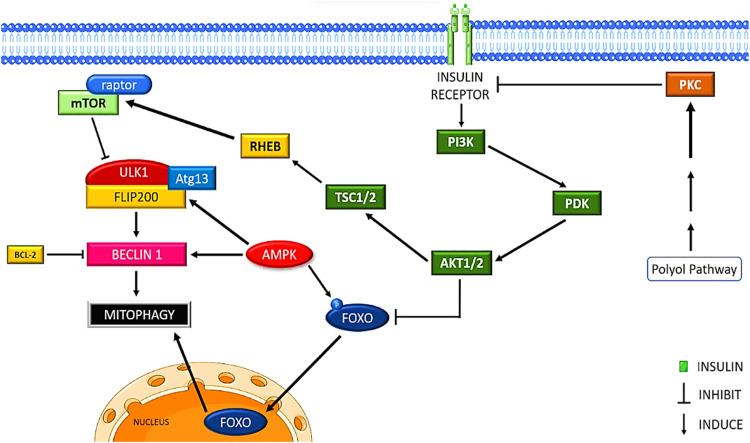
A major source of production of intracellular ROS as well as a key target of oxidative damage in diabetes, are the mitochondria. As the number of damaged mitochondria starts building up (mostly as a result of decreased mitophagy), ROS production rises through a process called ROS-induced ROS release (RIRR), creating a malicious cycle that eventually results in cardiomyocyte mortality. Both T1D and T2D patients typically exhibit increased mitochondrial ROS generation as well as impaired mitochondrial structure and function in their hearts [80]. The PI3K/AKT/mTORC1 pathway is activated by insulin (Fig. 3). Thus, it was initially hypothesized that T1D and T2D would both have elevated levels of mitophagy. However, mitophagy is actually found to be suppressed in T1D animal hearts [81].
Fig. 3.
Insulin receptor activation inhibits mitophagy via PI3K/PDK/AKT pathway, FOXO translocation prevention, mTORC1 activation and polyol pathway interference.
Owing to the increased mTORC1 activity and/or reduced AMPK activity, various studies have hypothesized the suppression of mitophagy in the T1D heart [82]. Though T1D is associated with mitochondrial dysfunction, studies have found that mitophagy proteins PINK1 and PARKIN are significantly reduced in diabetic hearts. Despite the fact that T1D is linked to mitochondrial dysfunction, research has shown that the levels of the mitophagy proteins PINK1 and PARKIN are considerably reduced in diabetic hearts. This shows that, despite severe mitochondrial dysfunction, PINK1/Parkin-mediated mitophagy is decreased in these hearts, causing an accumulation of malfunctioning mitochondria and the induction of cell death in myocytes [83]. However, according to another study, altered Rab9-mediated mitophagy has been seen to be correlated with cardiac injury [76]. Although there is clear evidence that cardiac mitophagy is reduced in T1D, the functional aspect of this reduction in mitophagy is still not clear and highly debatable [7].
Unlike T1D, results from studies on T2D are largely inconsistent. In T2D, cardiac mitophagy can remain unchanged, increased, or even reduced [84]. One of the reasons for such a diversified outcome of mitophagy in T2D is that the microenvironment of T1D is different from that of T2D. T2D is correlated to obesity, which comes along with a series of risk factors such as hyperglycemia, elevated levels of low-density lipoprotein and triglyceride circulation, coronary artery disease, and hypertension [85,86]. Hyperglycemia often leads to osmotic stress through the production of sugar alcohols, or ‘‘polyols’’. Under such hyperglycemic conditions, sorbitol aggregates within cells and facilitates osmotic stress. Additionally, excessive flux through the polyol pathway has been found to promote the production of ROS in the mitochondria [87]. Long-term consumption of an excessive high-fat diet (HFD) frequently causes obesity and insulin resistance [88]. Thus, to replicate the physiological conditions during T2D, HFD-induced mice are often used for studies. Sciarretta et al. observed that HFD activates the Ras homolog enriched in brain (RHEB) protein in cardiac myocytes, which in turn promotes mTORC1 activation and thus ultimately suppresses mitophagy. Furthermore, it was discovered that HFD-fed mice had increased p62 and decreased LC3II levels [89]. Contrarily, Xu et al.’s study revealed autophagic flux associated with insulin resistance and metabolic syndrome. They also observed that HFD-fed mice experience cardiac function impairments and accumulation of LC3II in their hearts, which suggests an elevated amount of autophagosomes [90].
CM is a cardiomyocyte-specific disease characterized by impaired myocardial structure and function. Anatomical dissection of hearts revealed a form of abnormal myocardial characteristics, left ventricular hypertrophy (LVH) and diastolic dysfunction being the most common clinical features [91]. However, these abnormalities are also sometimes found to be associated with many forms of heart failure. Thus, in order to distinguish these patients from patients with other varieties of CM, the term DCM was proposed. The diabetic heart muscles' decreased efficiency is caused by multiple mechanisms [92]. They include increased fatty acids and cytokines, as well as hyperglycemia. It has been found that hyperglycemia is maladaptive and increases the enzymatic O-GlcNAcylation of cardiomyocyte proteins. Increased chemical nonenzymatic advanced glycation end-product formation also has adversities. Impaired insulin signaling is a defining feature of both T1D and T2D. Other signaling cascades are altered in DCM, especially with increased protein kinase C (PKC) and MAPK signaling and reduced AMPK (AMP-activated protein kinase) signaling to have unfavorable effects [93,94].
In the case of IHD, myocardial damage is the result of inadequate myocardial requirement and coronary blood flow. Ischemia promotes myocardial cell death and cell damage. Ischemia/reperfusion (I/R) injury, caused by reperfusion, leads to irreversible heart damage [95]. An increased imbalance of mitophagy is caused by I/R due to I/R-induced damage to mitochondrial cristae, abnormalities in mitochondrial membrane potential, and mitochondrial permeability transition pore (mPTP) [96]. Mitophagy prevents cardiomyocytes from I/R injury [97]. Mitophagy has a protective role in cardiac myocytes. It also takes part in the coordination of Parkin recruitment and Drp1-mediated mitochondrial fission. Inhibiting mitophagy may alleviate cardiomyocyte I/R damage, lower apoptosis, and even enhance cardiac function [98]. In I/R injury, PINK1 and Parkin are upregulated, and Parkin translocation and activation are elevated. PINK1-ablated mice display aberrant mitochondrial function, pathological hypertrophy, and increased oxidative stress, while Parkin-depleted mice show higher cardiac remodeling [99]. Therefore, the progression of myocardial hypertrophy and remodeling are adverse outcomes of activation and inhibition of mitophagy. There is an ardent requirement for vast studies to demonstrate the role of mitophagy in IHD pathology in detail.
HF is also characterized by mitochondrial dysfunction. HF is eventually caused by the build-up of oxidative damage to mitochondrial proteins, lipids, and DNA due to decreased mitophagy-related clearance of damaged mitochondria. Several studies have revealed reduced expression of mitophagy-specific genes such as Beclin1 and LC3-II in the specimen of patients with HF. MFN2 has been demonstrated to alter the mitochondrial membrane potential by regulating HF-associated mitophagy [100]. Further research has revealed that heart injury is often found to be elevated due to ineffective mitophagy. Additionally, PINK1 downregulation is mostly associated with decreased mitophagy in patients with HF [101]. However, the cause and effect of mitophagy in HF remain controversial. Recent shreds of evidence also focus on the role of ULK1-dependent mitophagy against HF, but further research is needed for understanding its role in HF [102]. During mitophagy, mitochondrial DNA (mtDNA) also gets degraded in lysosomes by DNase II. It has also been reported that incomplete or partial digestion of mtDNA triggers inflammation in cardiomyocytes, which can ultimately lead to HF [103]
In diabetes, elevated ROS production and induced mitochondrial dysregulation in the heart result in cardiomyocyte death. Reduced mitophagy accumulates damaged mitochondria, causing a vicious cycle of ROS production. Poly-ubiquitinated chains trigger recruitment of both ubiquitin-proteasome and mitophagy machinery for degradation via mitophagosome formation. Understanding this pathway is crucial for exploring mitophagyʼs role in diabetic hearts and targeting its molecular components for therapy. T1D animal hearts have suppressed mitophagy. T2D results are inconsistent; hyperglycemia and a high-fat diet can suppress it, causing mitochondrial dysfunction. Mitophagy disruptions negatively impact mitochondrial homeostasis and contribute to CVD. Recent research suggests mitophagy functions as a cardioprotective mechanism, which is unclear in the diabetic heart [80,104].
4. Nano-biosensor-mediated diagnosis in diabetic heart condition
Detailed and accurate diagnosis is essential for properly treating the diseased individual. Early diagnosis and monitoring tests could spot the ones who require lifestyle alterations or pharmaceutical approaches to control the blood glucose level or delay the onset of diabetes and other associated cardiac problems. Substitution of conventional biochemical assays with cardiac biosensor devices facilitates prompt diagnosis. Traditional diabetes management techniques include continuous glucose monitoring (GM) and insulin administration, which have a significant influence on the lifestyle of the patients. The significant increase in the number of individuals suffering from DHC serves to highlight the shortcomings and ineffectiveness of the existing diagnosis and therapeutics of the disease. Emerging breakthroughs in nanotechnology have the potential to provide innovative diabetes monitoring strategies as well [105].
Conventional theranostic approaches can be reviewed and advanced with the assistance of nano-biosensors by offering rapid and early supersensitive diagnosis, prolonged assessment of potential biomarkers, point–of–care diagnostic tests, and in-prompt therapies [106,107]. Nano-biosensors are analytical systems that integrate a biological element with a biochemical detector for the diagnosis and treatment of DHC. They are highly preferable in the biomedical industry majorly due to their precision, sensitivity, efficiency, and specificity [108].
Electrochemical and optical biosensors are the most significant diagnosis systems as they possess all the desired properties for point–of–care diagnosis. Electrochemical biosensors offer a potential point-of-care technique in medical diagnosis owing to their inexpensive cost, convenience in handling, great sensitivity, and desirable specificity [109]. For the diagnosis of DHC, novel electrochemical biosensors featuring high electron/ion transfer rates, maximum adsorption potentials, as well as selective biomolecule immobilization were designed when nanomaterials and specified biomolecules were coupled [110]. When an immunogenic reaction occurs on the electrode surface of the biosensor, the current, resistance, or potential of the biosensor gets altered and this aids in the precise diagnosis of the suspected disease. Based on the principle of detection, electrochemical biosensors can be categorized as impedimetric, amperometric, potentiometric, and voltammetric biosensors [111,112]. An impedimetric biosensor detects and analyses the target molecule or biomarker and generates the output of the electrical impedance that directly corresponds to the activity of the target compound [113]. This is followed by the amperometric biosensor, which is an independent integrated system that diagnoses a disease by the output current resulting from oxidation and also provides a precise quantitative analysis. By evaluating the obtained results acquired for the multiple analyte concentrations, the selectivity of the amperometric biosensor is ascertained. A potentiometric biosensor operates on the basis of the potential difference between the reference electrode and the functional electrode. When compared to the reference electrode, its activity demonstrates a response that is proportional to the analyte concentration. Lastly, the voltammetric biosensors quantify the current that results from changing potential in order to receive information about an analyte [114]. Optical biosensors possess the ability to detect optical signals and have gained much attention in the biomedical field attributed to the tremendous advancements in nanotechnology and photonic elements. The identification of the biomarkers for diabetes and its associated DHCs have lately been widely examined using optical biosensors that follow the principle of fluorescence, colorimetry, chemiluminescence, resonance, Raman scattering, refraction, and photon compounds and have the ability to change the color when triggered by the target molecule [110]. With regard to uninterrupted real-time monitoring, tag or probe-free, electromagnetic interference-free, and intrinsically optimized GM units, optical biosensors are preferred over the widely available technologies for the estimation of biofluids, thus aiding the diagnosis of diabetic heart diseases. The target molecule is marked with a chromogenic or fluorescent tag during colorimetry and fluorescence-based detection. The visible alterations in the fluorescence or chromogen signal intensity signify the possible existence of the target compounds. Additionally, the method is highly precise, featuring a detection threshold of just one molecule [115].
Regardless of how crucial and significant the nano–biosensors are, the utility of nanomaterials in the biomedical industries always creates room for the adverse toxicological effect of the nanomaterials on the health of the patients in the long run. The nanotechnology-based medical sector thus still has a lot of scope for further research being conducted to provide better healthcare facilities to mankind. Therefore, Table 1 demonstrates the major advances made in of developing nanomaterial-based biosensors for detecting and diagnosing DHC-based biomarkers.
Table 1.
Tabular representation of recent advances in nano-biosensing based targeted diagnosis of the DHC using pathophysiological biomarkers.
| Nanosystem | Biomarker | Limit of Detection | Detection Principle | Disease | Ref. |
|---|---|---|---|---|---|
| FeMoOxNP@ABTS@DSPE-PEG | Hydrogen Peroxide | 2.5 µM | Optical biosensor sensitive towards near-infrared region | T2D | [116] |
| rGO-polyacrylamide-ferrocene/AuNPs | 1,5-anhydroglucitol | 21.74 µg/ml | Graphene oxide doped potentiometric sensor with high sensitivity | Diabetes | [117] |
| Luminescent Fe-carbon dots | Hydrogen peroxide and glucose | 3.86 µM (colorimetry) and 7.27 Μm (fluorometry) | Dual optical biosensor using colorimetric and fluorometric assay | Diabetes | [118] |
| 2-D SnS2 and 1-D Multi wall carbon nanotube | Cardiac troponin I | 0.02 fg/ml | Voltage-sensitive electronic sensor enabled with ML algorithm | CVD, DCM | [119] |
| LSPR based Gold nanorod conjugated with aptamers of target protein | C-reactive protein | 2 nM | Optical biosensor sensitive to UV absorption, resulting in LSPR shift | CVD | [120] |
| Polypyrrole nanotubes conjugated with NiCu layered double hydroxides | Glucose | 66 nM | Nonenzymatic biosensors having high electrocatalytic activities | Diabetes and heart diseases | [121] |
| Co3S4 hybridised graphite carbon nitride nanosheets | Hydrogen peroxide | 70 nM | Nonenzymatic amperometric biosensor possessing catalytic activity | CVD | [122] |
| Cu2O NP doped with (ChOx/TH/GCE) | Cholesterol | 0.0018 nM | Electrochemical biosensor utilising enzymatic immobilisation technique | CVD | [123] |
| GO/PPy/PANI/ZnO nanocomposite | Cholesterol and Bilirubin | 0.92 and 0.2 µA/µM/cm2 | High conductivity electrochemical biosensor | CVD | [124] |
| g—C3N4@Au NPs | Galectin-3 | 0.025 pg/ml | Amperometric immunosensor based on antigen-antibody reaction | DCM | [125] |
| NG/AuNPs and MoS2/AuPtPd nanodendrite | Growth Differentiation Factor-15 | – | Sandwich-type electrochemical immunosensor | DCM | [126] |
| Biofunctionalized silicon nitride doped with ion-sensitive Field effect transistor | N-terminal pro-brain natriuretic peptide (BNP) | 0.02 pg/ml | Electrochemical impedance spectroscopy | CVD (Heart failure) | [127] |
| Zn-MgO Nano-flakes | Glucose | 100 µl/ml | Enzymatic assay based on physical absorption method | Diabetes | [128] |
| AuNPs conjugated with Co3O4 nanorod transistor | Cardiac troponin T | 0.1 µg/ml | Non-labelled field-effect transistor based on immobilisation of the complementary biotinylated DNA aptamer via drop-casting method | CVD (Acute myocardial infarction) | [129] |
| CeO2-DNA nanosensor conjugated with Folic acid and CD36 antibody | Hydrogen Peroxide | 120 nM | Non-invasive opto-fluorescent nanosensor | CVD (Atherosclerosis) | [130] |
| Semicarbazide modified AuNPs, AgNPs, MOFs | N-terminal pro-B-type natriuretic peptide | 0.11 pg/ml | Immunoassay based on electrochemiluminescence resonance energy transfer | CVD (Heart failure) | [131] |
| Graphene quantum dot 3D ordered macroporous ZnO unit | Respiratory gaseous Acetone | 8.7 ppb | High affinity-based semiconductor doped zinc oxide biosensor towards trace gaseous biomarker | Diabetes | [132] |
5. Conventional therapeutic interventions associated with DHC
With the increasing disease awareness, diabetes has emerged as the primary cause of severe economic and social catastrophes affecting nations worldwide. The disease is still a matter of concern despite scientific advancements, improved healthcare services, and an increased proportion of literature, especially in emergent nations. Deathblows are escalating, as reported by the latest projections, posting a menace to global growth [133]. Sulphonylureas, biguanides, alpha-glucosidase inhibitors, and thiazolidinediones are examples of the latest generation of medications with enhanced efficiency in decreasing hyperglycemia that have been developed as a result of advancements in science and technology [134]. Nanocarriers are innovative drug-delivery systems that have been developed in response to recent advances in drug discovery [21].
T1D treatment involves increasing islet viability and cell regeneration via islet neogenesis-associated protein (INGAP) prophylaxis, insulin administration to help requite for beta cell deformities, dipeptidyl peptidase-4 (DPP-4) inhibition by sitagliptin, and much more [135]. Sulfonylureas and repaglinide endorse an insulin framework in hepatocytes, and metformin and troglitazone encourage insulin synthesis in both fat and muscles [136,137]. Miglitol and acarbose impose deferred sugar assimilation dietary patterns, and sulfonylureas and repaglinide increase insulin production [138]. The medications taken to treat T1D and T2D have certain drawbacks due to their serious adverse effects. The Food and Drug Administration (FDA)-approved therapeutic approaches of insulin with metformin, which lowered the routine insulin intake. A unified therapy of insulin with sulfonylureas, minimized weight gain allied with insulin therapy, and troglitazone-insulin combination effectively decreased insulin needs as well as improved blood sugar control are the other main therapies and practices [139,140]. Given that T2D individuals are already overweight, the use of thiazolidinedione is also linked to weight increase, which is a cause for concern. Drugs from the most recent generation, such as incretin mimics, can cause nausea, vomiting, and diarrhoea. The medications that have the potential to cure diabetes have been administered alone, in conjunction with drugs taken orally, but it is difficult to achieve comprehensive glycaemic control [138,140].
CM is a side effect connected to both T1D and T2D. Despite the lack of supplementary heart failure risk factors, including high blood pressure, coronary artery disease, atherosclerosis, or valve disease, CM can nevertheless lead to cardiac arrhythmia. The remodeling of the heart, pathologically is characterized by left ventricular eccentric hypertrophy and perivascular/interstitial fibrosis, contributing to the dysfunction of the organ [141]. Inhibitors of angiotensin-converting enzymes and blockers of angiotensin receptors which significantly reduce the likelihood of myocardial fibrosis and rigidity of left ventricles, which could stop and frequently undo the systemic and functional changes that happen even during the emergence of cardiomyopathy. Aldosterone antagonists reduce ischemic injury and address the antioxidant pathway as a treatment for DCM [142,143]. Itʼs worth noting that a number of medications being used today can enhance cardiac health in addition to glycemic control. Alongside their ability to reduce blood sugar levels, GLP-1 receptor agonists, besides sodium-glucose co-transporter 2 inhibitors (SGLTs), have been proven to benefit the cardiovascular system by directly having an impact on the myocardium [144].
The emerging patterns in diabetes therapies and supervision have unveiled the imperative need for scrupulous research aimed at testing composites and their counterparts in clinical trials (Table 2). The conventional intervention orthodoxy is challenged via clinical trials using novel anti-diabetic drug classes that show cardiovascular benefits despite very minor glucose lowering and point to the mechanism of action associated with a particular molecular pathway targeting autophagy as a key factor in outcomes [145].
Table 2.
Tabular representation of current anti-DHC drugs under clinical trials with severe limitations.
| Class of drug | Drug | Clinical trial Phase and ID | Targeted disease | Function | Drawbacks | Ref. |
|---|---|---|---|---|---|---|
| DDP-4i and SGLT2i | Lobeglitazone | Phase 4 active (NCT02285205) |
T2D | Boost glycemic and lipid control, cut back insulin resistance | Weight gain, edema, congestive, and heart failure chances | [146] |
| PPARs agonist | Chiglitazar | Phase 3 active (NCT02173457) |
T2D | Manage glucose concentration, insulin reactivity, and fat depletion | Edema and weight gain | [147] |
| PPAR agonists | Gemcabene Calcium | Phase 2 active | CVD | Improving lipid profile | Risk of hepatotoxicity | [148] |
| SGLT2i | Empagliflozin | Phase 4 (NCT03485092) |
CVD | Promote glucose elimination through the urine and inhibit reuptake by the kidneys | Hypotension, ketoacidosis, acute kidney, and hypoglycemia | [149] |
| SGLT2i | Canagliflozin | Phase 3 (NCT01064414) |
CVD, T2D | Glucose re-absorption, lowers body weight and blood pressure | Urinary tract infections and Hypotension | [150,151] |
| Monoclonal antibodies | Rituximab (anti-CD20), Abatacept (CTLA4-Ig) |
Phase 2 suspended (NCT03929601) | T1D | Insulin regulation | Upper respiratory tract infections, nasopharyngitis, bronchitis, sinusitis | [152] |
| GLP-1 | Tirzepatide | Phase 3 (NCT04537923) | T2D | Decrease glycemic levels, revamp insulin sensitivity, and enhance lipid metabolism | Nausea, diarrhoea or constipation | [153] |
| Neuropeptide | Substance P | Phase 1 (NCT02820558) | DCM | Regulate glucose and insulin sensitivity, and reduce fibrosis | Skin inflammation | [154] |
| DPP-4i | Gemigliptin | Phase 3 (NCT04255238) | T2D | Glycemic/lipid control and renal impairment | Urinary tract infection, diarrhoea, headache, cough, and hypertension. | [155] |
| DPP4i | Evogliptin | Phase 4 (NCT02587975) | T2D | Reduce hba1c levels and regulate glucose levels | Constipation, diarrhoea, hyper-sensitivity | [156] |
| SGLT2i | Sotagliflozin | Phase 3 (NCT05562063) (NCT02531035) (NCT03285594) |
CVD, T1D and T2D |
Decreases blood glucose, body weight, and blood pressure | Gastrointestinal side effects, including severe diarrhea | [157] |
| SGLT2i | Bexagliflozin | Phase 3 active (NCT03259789) |
T2D | Reduce hba1c levels, reduce body weight, blood pressure | Causes diabetic ketoacidosis | [158] |
| DPP4-i | Teneligliptin | Phase 4 (NCT03231709) |
CVD, DCM | Improve blood glucose, reduce hba1c levels | Hypoglycemia and constipation, Upper respiratory tract infection, Nasopharyngitis | [159] |
| PPARα | Pemafibrate | Phase 3 (NCT03071692) | DCM | Lipid profile | Hyperlipidemia | [160] |
| Combined therapy of biguanide and statins | Metformin and Atorvastatin | Phase 3 (NCT02947620) | DCM | Inhibits the glucose uptake and synthesis of glucose in the liver | Hyperglycemia and Lactic acidosis | [161] |
6. Contribution of nanotechnology and nanocarrier-mediated drug delivery strategies for DHC-targeted autophagy and mitophagy
Nanocarriers can be broadly classified into two groups, viz. organic or biogenic cargos (and polymer-based) and inorganic or synthetic (metallic and carbon-based) nanocarriers. Organic nanocarriers or biogenic can be made into specific types such as liposomes, lipid nanoparticles, and exosomes by making use of their noncovalent interactions to exploit self-assembly and design. Recently, biomolecules such as DNA and proteins have been widely exploited to generate stimuli-responsive cargos for therapeutic interventions. Despite having advantages, they have limited success because of their disadvantages, including high toxicity and low amounts of active drug transport, which supersedes their advantages [162,163].
There are multiple studies that can effectively emphasize the therapeutic potential of nanoparticles and nano-conjugated systems on diabetes and associated diseases. Referring to a study by Ul Haq et al., the green synthesized silver nanoparticles (AgNPs) produced by Phagnalon niveum methanol extract showed promising results as an anti-diabetic treatment option. Diabetes mellitus (DM) was induced in 8-week-old albino Wistar rats weighing about 140–150 g. 200 mg alloxan per kg of the rats’ body weight was injected intraperitoneally for this purpose. Rats were divided into 5 groups viz. Group C: untreated, control rats, rats received only distilled water; Group DAC: diabetic control, rats received only alloxan; Group DG: glibenclamide treated diabetic rats; Group DE: P. niveum extract treated diabetic rats; Group DagNPs: AgNPs synthesis from P. niveum diabetic rats. The rats were sacrificed after 21-d administration. Oral glucose tolerance test, blood glucose levels, and urine sample analyses were carried out. It was noticed that there was a remarkable reduction in blood glucose levels when AgNPs were administered. It was also seen that the body weight had increased, and other profiles, such as liver, lipid, and kidney, had a good improvement [164].
T2D is on the rise worldwide. It is related to many factors leading to CVD, such as obesity, hypertension, and insulin resistance. T2D also leads to the formation of ROS [165]. In a study conducted by Díaz-Pozo et al., patients were recruited as per the hospital protocols. The inclusion criteria were 40–70 years of age with a history of diabetes over 10 years. The exclusion criteria were renal problems, smokers, alcoholics, obesity, and those who are at CVD risk. Gold-ceria NPs (Au/CeO2) were used in this study to assess their effectiveness in T2D patients. Gold loadings were varied as 10%, 4.4%, 1.79% and 0.82% (wt) in the mixture for identifying the optimal Au loading, which maintains the particle size and, at the same time, maintains the number of sites for catalytic activity. It was observed that Au/CeO2 nanoparticles at 0.82 % gold concentration showed promising anti-inflammatory as well as antioxidant properties in the leukocyte-endothelium interaction. This suggests that if the patient is at a risk of developing any CVD, these nanoparticles will be protective in nature. These results correlated with the other cell viability assays carried out [166]. Coronary microcirculatory dysfunction (CMD) gives rise to DCM. The efficacy of polysaccharide sulfate (PSS) loaded poly lactide-co-glycolide (PLGA) nanoparticles for the treatment of DCM was studied by Gao & Liang. Rats were administered with PSS, PSS-PLGA NPs, and saline for a period of 8 weeks through intraperitoneal injection. Echocardiography was conducted, and heart tissues were analyzed for their histology. It was studied that the PSS-PLGA NPs improved cardiac function and improved symptoms of DCM. This is a promising basis for the treatment of CMD and DMC [167].
HF in diabetes patients is mainly attributed to CM and fibrosis. It is also studied that most of the available anti-diabetic drugs fail to protect T2D patients against heart problems [168]. Certain drugs for DCM, such as propylene glycol alginate sodium sulfate, have a higher molecular weight and are unstable, which hinders its clinical administration. This problem was solved using nanoparticles such as propylene glycol alginate sodium sulfate embedded in poly lactic-co-glycolic acid. RAGE expression was higher in the propylene glycol alginate sodium sulfate -nanoparticle-treated group than in the non-treated group in STZ-induced diabetic mice [169]. Another study utilized rat models of DCM to determine the effect of Calcium phosphate nanoparticles (CaPs) loaded with a mimetic peptide, which was administered through inhalation. After inhalation, the Cy-7 signals accumulated in the lungs and then crossed the lung barrier to reach the heart, where the peptide was released. When the peptide release peaked in the heart, improved contraction of the heart was also observed, providing cardiac protection to the rats. This demonstrates that inhalation of these therapeutic peptide nanocarriers is an effective approach to tackling DCM [170].
The currently available preclinical data demonstrate a limited number of nanotherapies targeting autophagy or mitophagy for mitigating or managing diabetes and DCM. Thus, the need of the hour is to utilize the nanoparticles and nano-conjugated material to combat diabetes and DCM as they have better biocompatibility and are much safer in comparison to conventional methods of therapeutics [171]. The use of nanomedicine also addresses problems like systemic toxicity, sudden immune activation, and thrombosis, wherein nanodrugs can control drug release and increase efficiency. They also eliminate problems of bioavailability, solubility, and pharmacokinetics [172,173]. The upcoming segments effectively and descriptively emphasize the potential applications of each biogenic and non-biogenic nanocargos in the therapeutic interventions of DHC and associative ailments, focusing specifically on autophagic and mitophagic targets.
Numerous established and investigational treatments that can potentially positively or negatively influence autophagy have been extensively worked upon. To quote a few studies, Battiprolu et al., demonstrated abnormally high activities of FOXO1 in the hearts of HFD-induced and genetic T2D mice models, which is shown to be a considerable contributor to DCM development [31]. To date, a battery of FOXO inhibitors like AS1842856 (AS) and N-[3-(1H-1,3-benzodiazol-2-yl)-1H-pyrazol-5-yl]-4-(4-methylpiperazin-1-yl)benzamide and others have shown to play a positive role in regulating glucose levels in diabetic mice [174,175]. Furthermore, resveratrol modulates the switching of cardiac cells between autophagy and apoptosis in DCM by regulating AMPK-mediated phosphorylation of mTORC1/p70S6K1/4EBP1 and the subsequent dissociation of Beclin1-Bcl-2 mediated by JNK pathway [176]. Aldose reductase catalyses the rate-limiting step of the polyol pathway, but its activation in hyperglycaemic conditions leads to diabetic complications, making it a prominent drug target for the treatment of diabetic complications [177]. Various aldose reductase inhibitors have shown great potential in ameliorating diabetes and diabetes-induced cardiac dysfunctions in preclinical studies [178] by targeting autophagy in diabetic hearts. Aldose reductase inhibitors function by delimiting the anomalous activation of autophagy caused by the activation of FOXO that is mediated by a reduction in Akt activity that leads to an increment in the autophagy genes [178,179]. A polyphenolic compound, sinapic acid, has also been reported to ameliorate STZ-induced DCM by mitigating increased glucose and lipids levels by regulating NRF2/HO-1 and NF-κB pathways [180].
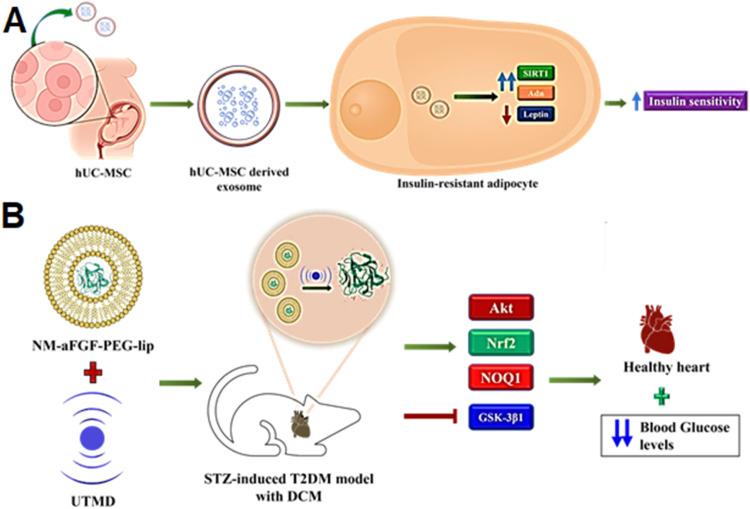
The pioneering work describing the potential of extracellular vesicles for ameliorating CVD came into circulation in 2010 [181], which is a result of the indirect contributions of the engrafted cells that release the cardioprotective paracrine factors, notably the EVs. The cardiac progenitor cell (CPC) [182] and mesenchymal stem cells (MSC) derived exosomes have shown recognizable downregulation of the apoptotic signals and reduction of the infarct size, thus revamping the myocardial functions [183]. Similarly, many pre-clinical studies have assessed the therapeutic index of EVs for treating T1D and T2D [184]. Chen and coworkers utilized exosomes emanated from human umbilical cord mesenchymal stem cells (hUC-MSC) that enhanced insulin sensitivity in adipocytes resistant to insulin. Treatment with exosomes upregulated SIRT-1 mRNA and adiponectin (Adn) while downregulated leptin expression, ultimately leading to the modulation of adipocytokines, making the hUC-MSC-derived exosomes a potential ameliorative agent for treating T2D and the associated pathologies (Fig. 4A) [185].
Fig. 4.
Nanoparticle and nanocarrier-based drug delivery for diabetes and related conditions targeting autophagy and mitophagy. (A) hU-MSC-Derived Exosomes enhance insulin sensitivity in insulin-resistant adipocytes through the regulation of SIRT1, Adn and Leptin. (B) PEGylated liposomes containing aFGF, delivered using UTMD, activate the Akt pathway, inhibit GSK-3β1, and increase Nrf2, NOQ1 and SOD2 expression to protect against oxidative-stress induced damage.
Zhang and colleagues employed a novel strategy to modulate autophagy by utilizing acidic fibroblast growth factor (aFGF). This growth factor exhibits a robust mitogenic potential and is recognized for its antiapoptotic, cardiogenic, and vasoactive attributes. These properties are conducive to enhancing cellular survival in scenarios involving DNA damage, oxidative stress-induced damage, or ischemia/reperfusion (I/R) injury. aFGF is also known for reversing insulin resistance in the case of T2D and reducing blood glucose levels. Thus, recombinant non-mitogenic aFGF encapsulated in PEGylated nanoliposomes was formulated by Zhang and coworkers which were delivered using ultrasound-targeted microbubble destruction (UTMD) technique wherein the shear stress generated by the ultrasonic waves (12–14 MHz) allowed the release of the drug into the cardiomyocytes from the liposome (lips) formulation (Fig. 4B). Thus, utilizing the versatility and non-toxicity of these self-assembling amphipathic molecules with appreciable biocompatibility [186]. NM-aFGF-PEG-lips + UTMD treatment restored the systolic cardiac functions in male Sprague-Dawley rats with STZ-induced T2D and subsequent DCM. Furthermore, nano-liposome combination therapy also ensured the inhibition of TGF-β1 mediated cardiomyocyte fibrosis by minimizing the production and deposition of collagen in the cardiac tissues of diabetic rats and also shrinking the chances of cardiac apoptosis. The treatment also activated the Akt pathway by inhibiting GSK-3β1 and increasing Nrf2, NOQ1 and SOD2 expression, creating a shield against the oxidative stress in the DCM rat [187].
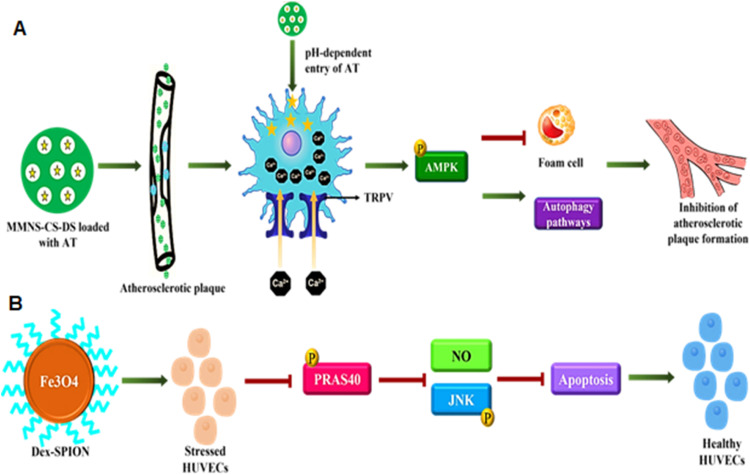
Another class of nanoparticles, the metallic nanoparticles (MNPs), as drug delivery systems hold multiple advantages like thermodynamic stability, enormous surface energies, and a large surface-to-volume ratio, which gives them a characteristic behavior wherein the surface atoms are thermodynamically more dominant and reactive than the internal atoms [188]. The use of metallic nanoparticles has been demonstrated in a study that was involved in developing a targeted therapy against atherosclerosis with a decent therapeutic index using pH-responsive chitosan (CS) based nano-carrier conjugated with dextran sulfate (DS), forming a ligand-specific to scavenger receptor type A that is overexpressed explicitly in the macrophages present in the atherosclerotic plaques. Magnetic mesoporous silica nanoparticles (MMSN) with high hysteresis regression values and an ability to cause a localized increase in temperature under an alternating magnetic field form the pedestal for the nanoparticle assembly. The MMNS-CS-DS loaded with atorvastatin (AT) targets the macrophages in atherosclerotic plaque in a pH-dependent fashion and also activates the thermosensitive Ca2+ channels called the transient receptor potential vanilloid subfamily 1 (TRPV1) that kindles the uptake of calcium ions and initiates the protective autophagy that reduces the lipoprotein build-up thus delaying the progression of the pathology. This dual-targeting MMNS-CS-DS-AT also possesses superparamagnetic properties that help in imaging using the magnetic resonance imaging technique, making it a theranostic nanoparticle (Fig. 5A) [189]. Similarly, an anti-TRPV1 antibody conjugated with copper sulfide nanoparticles was used as a photothermal switch for opening the TRPV1 cation channels that increase the intracellular concentrations of calcium ions, which stimulates the phosphorylation of AMPK to initiate the synthesis of the autophagic proteins implicated in the emergence of autophagosome and restrain the formation of foam cells for alleviating the lipid storage and AS plaque formation [190]. Of note, nanosized magnetic materials like iron oxide nanoparticles develop an internal magnetic field due to the presence of a core made up of magnetite or maghemite, which allows them to align themselves as per the external magnetic field, and Brownian motion randomizes this alignment in the absence of a magnetic field. However, magnetic materials exhibit this behavior of supramagnetism if and only if their sizes are reduced to a few nanometers. Thus, superparamagnetic iron oxide particles have been used in various targeted drug delivery and diagnostic systems [191]. Following the same principle, Duan and colleagues synthesized dextran-coated superparamagnetic iron oxide nanoparticles (Dex-SPION) to study the protective potential of the nanoparticles in human umbilical vein endothelial cells (HUVECs) against oxidative damage. Dex-SPIONs elevated autophagy response by reducing PRAS40 phosphorylation, a mTORC1 agonist responsible for upregulating NO production, thus inhibiting JNK phosphorylation and the subsequent apoptotic pathways, suggesting their role as adjuvant therapy for oxidative damage in DCM patients (Fig. 5B) [192].
Fig. 5.
(A) MMSN with CS-based nanocarrier conjugated with DS (MMNS-CS-DS) loaded with AT recognize the scavenger receptor type A on atherosclerotic plaque macrophage and ensure the rapid release of AT and the subsequent uptake of calcium ions via TRPV1 that can retard the formation of foam cells and the lipoprotein build-up, (B) Dex-SPION can upregulate autophagy response by downregulating PRAS40 phosphorylation and subsequent oxidative damage mediated apoptosis in DCM cell models.
Hydrogen sulfide (H2S), a gasotransmitter, is integral to cardiovascular homeostasis and also plays a myocardial protective function against myocardial ischemia-reperfusion (I/R) injury by mTOR activation, thus regulating autophagy [193]. Furthermore, the gasotransmitter inhibits apoptosis in I/R injury by regulating the miR-1-HDAC4 signaling pathway [194]. However, due to diverse side effects associated with the systemic delivery of H2S viz, central neurotoxicity, respiratory depression, etc. [195,196], The molecule could not reach the clinics, but by virtue of the cellular targeting capacity of nanoparticles, H2S-producing compounds (like Cystathionine-β-synthase (CBS), cystathionine-γ-lyase (CSE/CGL) and 3-mercapto-pyruvate sulfurtransferase (3-MST)) [197,198] achieved success in preclinical studies. Wang and coworkers developed a nano-delivery system for the localized cystathionine-γ-lyase (CSE) overexpression targeting I/R injury in rat models. The cardiac-specific nanodelivery system consisted of angiotensin-1 decapeptide (Gly-Gly-Gly-Gly-Asp-Arg-Val-Tyr-Ile-His-Pro-Phe) with terminal lysine covalently conjugated with PEGylated CdSe/ZnS quantum dots (PPQD) packaged with CSE DNA plasmid. CSE DNA plasmid nanocarriers with AT-1 (test) and without AT-1 (control) administered via jugular vein prior to reperfusion in I/R rat models when compared for their CSE levels in myocardial tissue depicted appreciable expression in the test group along with smaller infarct area and an increased ejection fraction than the control. Moreover, CSE expression in the control group's liver, lung and other tissues was equivalent to the myocardial tissue, but I/R rats administered with AT-1 conjugated PPQD depicted a significant gap. Notably, the synthesis of CSE ensured the inhibition of apoptotic pathways along with endoplasmic reticulum stress (ERS) induced mitophagy by a reduction in C/EBP homologous protein (CHOP), glucose‑regulated protein (GRP78) and eukaryotic initiation factor 2 a (eIF2a) production. Thus, the cardioprotective potential of H2S could be harnessed with target-specific nanocarriers that can eliminate the global effect and the accompanying disadvantages [199].
Mitochondria is one of the major sites for producing ROS, which tends to open mitochondrial permeability transition pores, and the sequelae of the pore opening, mitophagy, and autophagy pathways get activated [81]. To ameliorate the ROS-mediated autophagy, cardiomyocytic mitochondria targeting nanoparticles were developed by sequentially conjugating tempol (Tpl) and 4-(hydroxymethyl)phenylboronic acid pinacol ester (PBAP) to β-cyclodextrin (β-CD) decorated with stearyl triphenylphosphine (STPP) was developed by a team from Army Medical University, China. These ROS-scavenging NPs encase Ac2-26 (AMVSEFLKQAWFIENEEQEYVQTVK), an annexin/lipocortin 1-mimetic anti-inflammatory pro-resolving peptide [200]. The team utilized inhalation-based, non-invasive delivery of the therapeutic agent that employs the pulmonary circuit using transcytosis mediated by caveolae and/or paracellular transmission of the inhaled NP via a tight junction. The cyclic oligosaccharide-based NP also depicted intrinsic antioxidative and anti-inflammatory properties, which work synergistically with the Ac2-26 peptide that is delivered by the nanovehicle [201]. Thus, the alliance of polymer chemistry and nanotechnology have led to the development of polymeric nanoparticles with the ability to transform and potential for reabsorption in the body and ensure maximal effectiveness [202].
Tetrahedral DNA nanostructures (TDNs) are multifunctional biogenic nanoparticles formed by four strands of DNA that create a pyramid-like structure, which has been reported to have an antioxidant and antiapoptotic effect on DCM cell models. Post-administration, the TDNs were able to reduce ROS production and promote Akt phosphorylation that further activates the Akt/Nrf2 pathway, which is critical for boosting cardiomyocyte survival in case of I/R injury. pAkt ensures the separation of Nrf2 from an actin-anchored protein Keap1, and Nrf2 enters the nucleus and stimulates the expression of a cardioprotective enzyme heme oxygenase-1 (HO-1), forming a pragmatic approach in protecting against DCM [203].
7. Challenges in the utilization of nanotechnological therapeutic interventions
Since the dawn of nanotechnology, nanomaterials have been extensively used for studies involving applications in therapeutics. When nanoparticles encounter biofluids, they get exposed to different types of proteins, which leads to the formation of corona protein particles. Phagocytes of the immune system first interact with any foreign particles in the human body, including nanoparticles. This interaction can bring about immunosuppression or immunostimulation. Immunosuppression can make the person susceptible to a range of diseases, leaving the person incapable of fighting against the diseases, whereas immunostimulation leads to disorders such as inflammation [204]. Aggregation occurs when nanoparticles are tightly bound to each other, and it becomes extremely difficult to break them apart into their primary state. Unrestricted aggregation alters the functionality of nanoparticles. However, this can be advantageous in some cases, e.g., in 3D structures for photonic, Raman, and magnetic applications. On the other hand, agglomeration occurs when nanoparticles are weakly bound to each other. The main factors that trigger the immune response when a nanoparticle enters the body are size, shape, surface, and hydrophobicity. Once they enter the body, the innate immune system immediately responds to it as a foreign particle [205]. Biomedical properties of nanoparticles have attracted a lot of attention from researchers worldwide. However, their side effects and long-term toxicity are a cause of concern. Properties such as agglomeration, reactivity, impurities, and size led to the development of some risks for those exposed to nanoparticles or being administered NPs. Some of the acute toxic effects include the generation of ROS, denaturation of proteins, deviation of normal phagocytic functions, and mitochondrial discomfiting, whereas the chronic effects include organ dysfunction and enlargement caused by neoantigens, uptake by the nucleus, reticuloendothelial system, and neuronal tissue [206]. As per the toxicological data available, toxicity depends on various factors like dose and exposure time, aggregation, concentration, particle size, shape, surface area, crystal structure, surface functionalization and pre-exposure effect [206,207]. Another challenge in utilizing nanoparticles is their poor excretion and retention in the liver due to their large size. Smaller ones are cleared quickly by the lymphatic system. If the size of the nanoparticle is less than the glomerular filtration size limit, which is around 5.5 nm, it is eliminated via the kidney and is passed through the urine. If their size is larger than 5.5 nm, they will be held back for long periods of time in the Kupffer cells [208].
AgNPs are widely used as a therapeutic intervention for treating diabetes but are associated with certain risks and cell toxicity. Different cell lines demonstrate different toxicity results depending upon the dose concentrations [209]. A dose concentration of 50 µg/ml in mouse embryonic cells damages the DNA via p53 and DNA repair proteins Rad51 and H2AX upregulation, while in the human hepatoma cell line, a dose of 1 µg/ml is responsible for persuading cell toxicity [210]. However, in beige adipocyte cell lines, AgNPs repress the differentiation properties of the cells along with reduced mitochondrial activity when assayed on beige adipocytes [211]. These nanoparticles also caused the adipose tissue cells to undergo fatty acid oxidation, thus increasing the cell toxicity due to thermogenesis. The concentration of blood glucose can be regulated by thermogenic fat cells or beige adipocytes in order to treat diabetic cardiomyopathy using cell-based therapies [212]. Beige adipocyte cell lines stimulate the production of thermogenic responses with reduced obesity and hyperglycemia effects in mice models [213]. The energy produced after the oxidation of fatty acids by AgNPs additionally promotes the synthesis of reactive oxygen species (ROS), which is mainly responsible for causing cytotoxicity through the activation of the MAPK (mitogen-activated protein kinase) pathway [211].
Gold nanoparticles (AuNPs) are known for their applications in treating metabolic cardiomyopathies such as diabetic mellitus and heart failure [214]. Nevertheless, AuNPs have been proven to cause cytotoxicity depending upon the dose concentration, as demonstrated by a few in vitro experiments. The cytotoxic effect is spawned under oxidative stress on the cell translating into mitochondrial autophagy, activation of the MAPK pathway, and cell death [215]. A study in 2007 showed that about 91 % of the AuNPs were still accumulated in the liver even after 6 months. Nanoparticle clearance via the hepatobiliary system is slow and can range from some hours to even months [216].
Metal oxide nanoparticles such as zinc oxide nanoparticles (ZnO NPs) are considered effective therapeutic agents for the treatment of diabetes, however, ZnO NPs might induce cytotoxicity after a certain level of concentration [209]. ZnO NPs have been demonstrated to be one of the most toxic NPs to human cells, with the lowest LD50 values [217]. In an in vitro study, ZnO NPs were administered on human hepatocyte and embryonic kidney cell lines to evaluate the cytotoxicity effect. High concentrations of ZnO NPs cause the cells to change shape and induce oxidative stress and mitochondrial autophagy. The MTT assay results demonstrated cytotoxicity after the cells were treated with 10–100 µg/ml of ZnO NPs [218]. A few studies of diabetic rat models also indicated that administering ZnO in the amount of 100 mg/kg/d causes the cells to experience a hyperglycemic state [219]. The hyperglycemic state of a cell activates atherothrombosis in diabetic cells, which is linked with escalated morbidity and mortality rates in populations suffering from cardiovascular problems [220].
The use of nanoparticles has shot up in the last 10–15 years because of their many advantages and features. But it brings with it some disadvantages, such as cytotoxicity and its efficiency to be taken up by the body. To overcome these problems, the physicochemical properties of the NPs can be changed, and the surface can be functionalized to improve the uptake efficiency [221]. The use of an appropriate capping agent is a must for improving functionalization. Different types of capping agents can be used for this purpose. These are carbohydrates, proteins, amino acids, lipids, fungal extracts, bacterial extracts, algal extracts, and plant extracts. PEG has been most widely used to functionalize the NP surface to improve their biocompatibility and uptake. Dextran is used to modify nanoparticles to reduce their toxicity. Another polysaccharide used for this purpose is CS. Collagen, a natural animal protein, is used to cap nanoparticles. It exhibits good biocompatibility, degradation, self-assembly, and cross-linkage, which makes it one of the best protein-capping agents. Silver nanoparticles were prepared using collagen as a reducing agent and stabilizing agent. Varying concentrations of AgNO3 were mixed with the collagen gel. The effect of collagen on the size, shape, and stability was studied. It was observed that collagen prevented the formation of nanoparticle aggregation, controlled its size, and provided stability [206].
Addressing the bottlenecks in translating nano-based diagnosis and therapeutic strategies for diabetes is crucial for harnessing the full potential of nanomedicine in diabetes management. Navigating through regulatory frameworks and obtaining approvals for nano-based DHC therapies pose formidable challenges. Regulatory agencies require a robust understanding of the nanomaterial's safety profile, pharmacokinetics, and pharmacodynamics.
8. Future perspectives for DHC management
To tackle DHC, the focus must be given to the diagnosis and therapeutic strategies. In both of these strategies, nanotechnology plays a key role in the improvement of accuracy and sensitivity of diagnostic tests, potentially allowing earlier detection of the disease. For instance, nanoparticle-based contrast agents can be used to enhance the imaging of the heart in magnetic resonance imaging (MRI) and computed tomography (CT) scans, providing improved visualization of the heart's structure and function [222]. Additionally, nanosensors and nanodevices can be used to monitor biomarkers in the blood or tissues, providing real-time information about the progression of the disease [223]. Some of the biomarkers that can be targeted using nanotechnology are LC3-II, a lipidated form of the LC3 protein found on autophagosomes, the structures that engulf and degrade cellular components during autophagy. LC3-II levels are often used as a marker of autophagy activity. Studies show that LC3-II levels are increased in the sciatic nerves of diabetic rats in diabetic neuropathy and increased in the kidneys of diabetic mice in diabetic nephropathy. Mitophagy can also be detected using specific biomarkers. Nanobiosensors are thus powerful tools for detecting biomarkers associated with autophagy and mitophagy, including LC3-II, p62, Parkin and TOMM20. These biomarkers can be used to diagnose and monitor these diseases, as well as to evaluate the efficacy of therapeutic interventions [224]. Nanoparticle-based contrast agents can be developed to enhance the visualization of blood flow in Myocardial perfusion imaging, providing improved information about the heartʼs function. Nanoparticle-based sensors can be designed to detect endothelial dysfunction markers to provide a quick and sensitive assessment of blood vessel damage.
Biogenic and non-biogenic nanoparticles can be used for the diagnosis and treatment of diabetic heart. Biogenic nanoparticles can be engineered to target specific tissues or cells, such as those affected by diabetic heart disease, thereby increasing the efficiency and specificity of therapeutic intervention [225]. These nanoparticles can also be engineered to bind to specific autophagic and mitophagic biomarkers, such as specific proteins or metabolic products, and to fluoresce, providing a visual indication of their presence. This information can then be used to diagnose the disease or to monitor its progression over time. The efficacy of non-biogenic nanoparticles can be improved by improving the target specificity of nanoparticles by engineering the surface properties of nanoparticles with specific targeting moieties such as antibodies or peptides. This can be achieved by increasing the stability of nanoparticles via modifying their composition, size, or surface properties [226]. Enhancing the drug-loading capacity of nanoparticles or reducing the toxicity of nanoparticles by modifying their size or composition or by coating them with a layer of biodegradable materials [227].
Strategies using miRNAs can also help regulate diabetic heart disease. Numerous studies have demonstrated that alterations in the expression of specific miRNAs are associated with the onset of diabetic heart disease. By targeting these miRNAs with therapeutic agents, it may be possible to reverse or slow down the progression of this condition. One approach to miRNA therapy is to use miRNA mimics, which are synthetic RNA molecules designed to increase the expression of a specific miRNA. Another approach is to use miRNA inhibitors, which are designed to reduce the expression of specific miRNAs. Genomics and proteomics can also be utilized to develop therapies for this condition. Genomics-based therapies aim to target specific genetic mutations or variations that contribute to the development of DHC. Researchers may identify genetic markers that are associated with an increased risk of the disease and develop therapies that target these markers to prevent or slow down the progression of the disease [228]. Proteomics-based therapies aim to target specific proteins. Researchers may identify proteins that are involved in the development of inflammation or oxidative stress, which are two key factors in the development of diabetic heart condition. Further, development of therapies can be used to target these proteins to reduce inflammation or oxidative stress, thereby slowing down the progression of the disease [229].
Bioinformatics-based therapies can also be aimed at generating information that can be used to identify specific targets for therapeutic intervention, such as genetic mutations, protein dysregulations, or metabolic imbalances that contribute to the development of the disease. One approach to bioinformatics-based therapy is to use machine learning algorithms to predict which patients are at risk of developing this condition based on their genomic, transcriptomic, proteomic, or metabolic profiles [228,230]. This information can then be used to develop personalized prevention or treatment plans for these patients. Another approach is to use bioinformatics tools to predict which drugs or drug combinations are most likely to be effective in treating diabetic heart. This can be achieved by using computational methods to analyze large amounts of data on the mechanisms of drug action and the biology of the disease. Moreover, some studies show metabolomic-associated systemic biology studies, which reveal how metabolic functions during T2D impact myocardial redox roles [231]. Artificial intelligence (AI) and Machine Learning (ML) are becoming increasingly important in the field of diabetes and heart disease, as they have the potential to revolutionize the way we diagnose, treat, and monitor these conditions. Continuous glucose monitoring systems, which use AI algorithms to analyze blood glucose data, can help people with diabetes keep their glucose levels in check [232]. These systems use machine learning algorithms to predict glucose levels in real-time and provide users with accurate glucose readings, reducing the need for traditional finger-stick blood glucose testing. AI/ML can also be helpful in diagnosing and monitoring by utilizing it to identify new drug targets and to optimize the dosing and administration of existing drugs. Thus, AI/ML has enormous potential to revolutionize the way we manage diabetic heart, by providing more accurate, personalized, and effective care [233].
Smart nanomaterials have the potential to play a major role in the diagnosis and treatment of diabetic heart disease due to their ability to target specific cells or tissues in the body, thereby reducing the side effects associated with traditional treatments. For instance, nanoparticles loaded with drugs can be designed to seek out and target diseased cells in the heart, delivering a concentrated dose of medication where it is most needed. This targeted approach can increase the effectiveness of treatment and reduce the risk of adversities [234]. In terms of diagnosis, smart nanomaterials can be designed to detect disease markers in the blood or tissues, allowing for earlier and more accurate diagnoses [235]. Another area where smart nanomaterials have potential is monitoring treatment outcomes. Marinheiro et al., conducted a study on nanoparticles equipped with sensors that can be used in monitoring the effectiveness of drug therapy, providing real-time information on the level of drug delivery to the heart and its impact on disease progression [236]. This can help guide treatment decisions and ensure that patients receive the most appropriate and effective care. Smart nanomaterials also have the potential to improve our understanding of the underlying mechanisms of DHC. By using nanoparticles as tools for imaging and sensing, researchers can gain new insights into the disease process and identify new targets for treatment.
The continuous progress of nanobiosensors aims to improve diagnostic accuracy for diabetic heart disease. Advances in nanotherapeutics are being pursued to achieve increased effectiveness while minimizing toxicity. Integrating nanotechnology with precision medicine is paving the way for personalized medication. Additionally, enhanced imaging techniques, such as nanoparticle-based imaging, show potential for deeper insights into the biology of diabetic heart disease. Though these technologies are still in early development, they promise to transform the management and treatment of this challenging condition.
9. Conclusion
DHC and associated diabetic myopathies are reaching pandemic levels, and despite scientific advancements, the disease burden is rapidly increasing. This condition makes affected individuals susceptible to a range of debilitating complications, including heart failure primarily caused by impaired or overactive autophagy pathways. In diabetic hearts, inhibition of autophagy and mitophagy is the main cause of cardiac injury in T2D models, whereas it plays a protective role in Type 1 T1D models. This highlights the need for drug-based cardiac therapies that target autophagy and mitophagy pathways (such as AMBRA1, Parkin/PINK1, FUNDC1, BNIP3/NIX, Bcl2L13, CHDH, mTOR/AMPK, FOXO, SIRT, Beclin 1, and PI3/Akt) to regulate cardiac tissue repair, regeneration, and cardioprotective functions in diabetic hearts.
The necessity for targeted delivery of cardioprotective agents has brought nanoparticles into focus. Nanoparticles can enhance drug solubility, prolong drug half-life in tissues, and improve drug transfer across biological membranes and barriers. This not only maximizes drug bioavailability but also minimizes contact between healthy tissues and drugs. However, before a nanoparticle-based therapy can be used clinically, it must undergo rigorous preclinical and clinical trials. Nanoparticle formulations targeting autophagy and mitophagy in diabetic hearts are currently in the preclinical stage and require extensive pharmacokinetic and optimization studies to make cardiac regeneration a clinical reality. However, before a lead can be utilized as a therapeutic regimen, it must qualify the preclinical and clinical trials, and the nanoparticle concoctions targeting autophagy and mitophagy in diabetic hearts are still preclinical and require extensive pharmacokinetic and optimization studies to make cardiac regeneration a clinical reality.
CRediT authorship contribution statement
Sagnik Nag: Validation, Visualization, Writing – review & editing, Conceptualization, Supervision. Oishi Mitra: Validation, Visualization, Writing – review & editing, Conceptualization, Supervision. Bhanu Maturi: Supervision, Visualization, Writing – review & editing. Simran Preet Kaur: Validation, Visualization, Writing – review & editing. Ankita Saini: Validation, Visualization, Writing – review & editing. Muskan Nama: Validation, Visualization, Writing – original draft. Soumik Roy: Validation, Visualization, Writing – review & editing. Souvik Samanta: Validation, Visualization, Writing – review & editing. Leena Chacko: Supervision, Validation, Writing – review & editing. Rohan Dutta: Validation, Visualization, Writing – review & editing. Suresh Babu Sayana: Validation, Visualization, Writing – review & editing. Vetriselvan Subramaniyan: Supervision, Writing – review & editing. Jasvinder Singh Bhatti: Validation, Writing – review & editing. Ramesh Kandimalla: Conceptualization, Formal analysis, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Conflicts of interest
The authors declare that they have no competing/conflicting interests.
Contributor Information
Sagnik Nag, Email: sagniknag234@gmail.com.
Oishi Mitra, Email: oishi.mitra10@gmail.com.
Jasvinder Singh Bhatti, Email: jasvinder.bhatti@cup.edu.in.
Ramesh Kandimalla, Email: ramesh.kandimalla@gmail.com.
References
- 1.Rajbhandari J., Fernandez C.J., Agarwal M., Yeap B.X.Y., Pappachan J.M. Diabetic heart disease: a clinical update. World J Diabetes. 2021;12:383–406. doi: 10.4239/wjd.v12.i4.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghosh N., Chacko L., Bhattacharya H., Vallamkondu J., Nag S., Dey A., et al. Exploring the complex relationship between diabetes and cardiovascular complications: understanding diabetic cardiomyopathy and promising therapies. Biomedicines. 2023;11:1126. doi: 10.3390/biomedicines11041126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dewanjee S., Vallamkondu J., Kalra R.S., John A., Reddy P.H., Kandimalla R. Autophagy in the diabetic heart: a potential pharmacotherapeutic target in diabetic cardiomyopathy. Ageing Res Rev. 2021;68 doi: 10.1016/j.arr.2021.101338. [DOI] [PubMed] [Google Scholar]
- 4.Ritchie R.H., Dale Abel E. Basic mechanisms of diabetic heart disease. Circ Res. 2020;126:1501–1525. doi: 10.1161/CIRCRESAHA.120.315913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ormazabal V., Nair S., Elfeky O., Aguayo C., Salomon C., Zuñiga F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17:1–14. doi: 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sciarretta S., Maejima Y., Zablocki D., Sadoshima J. The role of autophagy in the heart. Annu Rev Physiol. 2018;80:1–26. doi: 10.1146/annurev-physiol-021317-121427. [DOI] [PubMed] [Google Scholar]
- 7.Zheng H., Zhu H., Liu X., Huang X., Huang A., Huang Y. Mitophagy in diabetic cardiomyopathy: roles and mechanisms. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.750382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uoselis L., Nguyen T.N., Lazarou M. Mitochondrial degradation: mitophagy and beyond. Mol Cell. 2023;83:1–17. doi: 10.1016/j.molcel.2023.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Gao J., Chen X., Shan C., Wang Y., Li P., Shao K. Autophagy in cardiovascular diseases: role of noncoding RNAs. Mol Ther Nucleic Acids. 2021;23:101–118. doi: 10.1016/j.omtn.2020.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Y., Li T., Li Z., Liu N., Yan Y., Liu B. Role of mitophagy in cardiovascular disease. Aging Dis. 2020;11:419–437. doi: 10.14336/AD.2019.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leon B.M., Maddox T.M. Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes. 2015;6:1246. doi: 10.4239/wjd.v6.i13.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinnen D., Kruger D., Magwire M. Type 2 diabetes and cardiovascular disease: risk reduction and early intervention. Postgrad Med. 2023;135:2–12. doi: 10.1080/00325481.2022.2126235. [DOI] [PubMed] [Google Scholar]
- 13.Ma Q., Ma H., Xu F., Wang X., Sun W. Microfluidics in cardiovascular disease research: state of the art and future outlook. Microsyst Nanoeng. 2021;7:19. doi: 10.1038/s41378-021-00245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayda S., Adeel M., Tuccinardi T., Cordani M., Rizzolio F. The history of nanoscience and nanotechnology: from chemical-physical applications to nanomedicine. Molecules. 2020;25:112. doi: 10.3390/molecules25010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahu T., Ratre Y.K., Chauhan S., Bhaskar L.V.K.S., Nair M.P., Verma H.K. Nanotechnology based drug delivery system: current strategies and emerging therapeutic potential for medical science. J Drug Deliv Sci Technol. 2021;63 [Google Scholar]
- 16.Chinchulkar S.A., Sankaranarayanan S.A., Rengan A.K. In: Nanobiosensors for point-of-care medical diagnostics. Gogoi M, Patra S, Kundu D, editors. Springer; Singapore: 2022. Nanobiosensor: advancement in disease diagnostic; pp. 257–279. [Google Scholar]
- 17.Yetisgin A.A., Cetinel S., Zuvin M., Kosar A., Kutlu O. Therapeutic nanoparticles and their targeted delivery applications. Molecules. 2020;25:2193. doi: 10.3390/molecules25092193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo X.M., Yan C., Feng Y.M. Nanomedicine for the treatment of diabetes-associated cardiovascular diseases and fibrosis. Adv Drug Deliv Rev. 2021;172:234–248. doi: 10.1016/j.addr.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Chandarana M., Curtis A., Hoskins C. The use of nanotechnology in cardiovascular disease. Appl Nanosci (Switz) 2018;8:1607–1619. [Google Scholar]
- 20.He Y., Al-Mureish A., Wu N. Nanotechnology in the treatment of diabetic complications: a comprehensive narrative review. J Diabetes Res. 2021;2021:1–11. doi: 10.1155/2021/6612063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su S., Kang P.M. Recent advances in nanocarrier-assisted therapeutics delivery systems. Pharmaceutics. 2020;12:1–27. doi: 10.3390/pharmaceutics12090837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galluzzi L., Bravo-San Pedro J.M., Levine B., Green D.R., Kroemer G. Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat Rev Drug Discov. 2017;16:487–511. doi: 10.1038/nrd.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang N., Zhou Y., Ngowi E.E., Qiao A. Autophagy: playing an important role in diabetes and its complications. Med Drug Discov. 2024;22:1–6. [Google Scholar]
- 24.Kobayashi S., Liang Q. Autophagy and mitophagy in diabetic cardiomyopathy. Biochim Biophys Acta Mol Basis Dis. 2015;1852:252–261. doi: 10.1016/j.bbadis.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 25.Hajra A., Bandyopadhyay D., Hajra S.K. Adenosine monophosphate- activated protein kinase- based classification of diabetes pharmacotherapy. J Postgrad Med. 2017;63:275. doi: 10.4103/jpgm.JPGM_434_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanamori H., Takemura G., Goto K., Tsujimoto A., Mikami A., Ogino A., et al. Autophagic adaptations in diabetic cardiomyopathy differ between type 1 and type 2 diabetes. Autophagy. 2015;11:1146–1160. doi: 10.1080/15548627.2015.1051295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meijer A.J., Codogno P. Signalling and autophagy regulation in health, aging and disease. Mol Aspects Med. 2006;27:411–425. doi: 10.1016/j.mam.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Yao F., Zhang M., Chen L. 5’-Monophosphate-activated protein kinase (AMPK) improves autophagic activity in diabetes and diabetic complications. Acta Pharm Sin B. 2016;6:20–25. doi: 10.1016/j.apsb.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartolomé A., García-Aguilar A., Asahara S.-I., Kido Y., Guillén C., Pajvani U.B., et al. MTORC1 regulates both general autophagy and mitophagy induction after oxidative phosphorylation uncoupling. Mol Cell Biol. 2017;37 doi: 10.1128/MCB.00441-17. e00441-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamargo-Gómez I., Mariño G. AMPK: regulation of metabolic dynamics in the context of autophagy. Int J Mol Sci. 2018;19:3812. doi: 10.3390/ijms19123812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Battiprolu P.K., Hojayev B., Jiang N., Wang Z.V., Luo X., Iglewski M., et al. Metabolic stress - Induced activation of FoxO1 triggers diabetic cardiomyopathy in mice. J Clin Investig. 2012;122:1109–1118. doi: 10.1172/JCI60329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paula-Gomes S., Gonçalves D.A.P., Baviera A.M., Zanon N.M., Navegantes L.C.C., Kettelhut I.C. Insulin suppresses atrophy- And autophagy-Related genes in heart tissue and cardiomyocytes through AKT/FOXO signaling. Hormone Metab Res. 2013;45:849–855. doi: 10.1055/s-0033-1347209. [DOI] [PubMed] [Google Scholar]
- 33.Kanamori H., Takemura G., Goto K., Tsujimoto A., Mikami A., Ogino A., et al. Autophagic adaptations in diabetic cardiomyopathy differ between type 1 and type 2 diabetes. Autophagy 2015;11:1146–60. [DOI] [PMC free article] [PubMed]
- 34.Marrif H.I., Al-Sunousi S.I. Pancreatic β cell mass death. Front Pharmacol. 2016;7:83. doi: 10.3389/fphar.2016.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loos B., Engelbrecht A.M., Lockshin R.A., Klionsky D.J., Zakeri Z. The variability of autophagy and cell death susceptibility: unanswered questions. Autophagy. 2013;9:1270–1285. doi: 10.4161/auto.25560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Escribano-Lopez I., Diaz-Morales N., Iannantuoni F., Lopez-Domenech S., de Marañon A.M., Abad-Jimenez Z., et al. The mitochondrial antioxidant SS-31 increases SIRT1 levels and ameliorates inflammation, oxidative stress and leukocyte-endothelium interactions in type 2 diabetes. Sci Rep. 2018;8:1–10. doi: 10.1038/s41598-018-34251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bairwa S.C., Parajuli N., Dyck J.R.B. The role of AMPK in cardiomyocyte health and survival. Biochim Biophys Acta Mol Basis Dis. 2016;1862:2199–2210. doi: 10.1016/j.bbadis.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Galluzzi L., Bravo-San Pedro J.M., Levine B., Green D.R., Kroemer G. Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat Rev Drug Discov 2017;16:487–511. [DOI] [PMC free article] [PubMed]
- 39.Munasinghe P.E., Riu F., Dixit P., Edamatsu M., Saxena P., Hamer N.S.J., et al. Type-2 diabetes increases autophagy in the human heart through promotion of Beclin-1 mediated pathway. Int J Cardiol. 2016;202:13–20. doi: 10.1016/j.ijcard.2015.08.111. [DOI] [PubMed] [Google Scholar]
- 40.Gatica D., Chiong M., Lavandero S., Klionsky D.J. The role of autophagy in cardiovascular pathology. Cardiovasc Res. 2022;118:934–950. doi: 10.1093/cvr/cvab158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chun Y., Kim J. Ampk–mtor signaling and cellular adaptations in hypoxia. Int J Mol Sci. 2021;22:9765. doi: 10.3390/ijms22189765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang L., Dai K., Chen M., Zhou W., Wang X., Chen J., et al. The ampk agonist pt1 and mtor inhibitor 3hoi-ba-01 protect cardiomyocytes after ischemia through induction of autophagy. J Cardiovasc Pharmacol Ther. 2016;21:70–81. doi: 10.1177/1074248415581177. [DOI] [PubMed] [Google Scholar]
- 43.Sciarretta S., Yee D., Nagarajan N., Bianchi F., Saito T., Valenti V., et al. Trehalose-induced activation of autophagy improves cardiac remodeling after myocardial infarction. J Am Coll Cardiol. 2018;71:1999–2010. doi: 10.1016/j.jacc.2018.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brillo V., Chieregato L., Leanza L., Muccioli S., Costa R. Mitochondrial dynamics, ROS, and cell signaling: a blended overview. Life. 2021;11:332. doi: 10.3390/life11040332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ding Y., Wang J., Lu J. miR-337-5p promotes the development of cardiac hypertrophy by targeting Ubiquilin-1 (UBQLN1) Bioengineered. 2021;12:6771–6781. doi: 10.1080/21655979.2021.1964892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Judith D., Jefferies H.B.J., Boeing S., Frith D., Snijders A.P., Tooze S.A. ATG9A shapes the forming autophagosome through Arfaptin 2 and phosphatidylinositol 4-kinase IIIβ. J Cell Biol. 2019;218:1634–1652. doi: 10.1083/jcb.201901115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tzifi F., Economopoulou C., Gourgiotis D., Ardavanis A., Papageorgiou S., Scorilas A. The role of BCL2 family of apoptosis regulator proteins in acute and chronic leukemias. Adv Hematol. 2012;2012:1–15. doi: 10.1155/2012/524308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pfeffer M.A., Shah A.M., Borlaug B.A. Heart failure with preserved ejection fraction in perspective. Circ Res. 2019;124:1598–1617. doi: 10.1161/CIRCRESAHA.119.313572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanki A., Gultekin N., Kucukates E. Evaluation of autophagy and microtubules inhibition through blood beclin-1 levels in subgroups with Heart Failure reduced Ejection Fraction. Clin Intervent Cardiol. 2021;1:1–11. [Google Scholar]
- 50.Jia L., Hao S.L., Yang W.X. Nanoparticles induce autophagy via mTOR pathway inhibition and reactive oxygen species generation. Nanomedicine. 2020;15:1419–1435. doi: 10.2217/nnm-2019-0387. [DOI] [PubMed] [Google Scholar]
- 51.Daneshgar N., Rabinovitch P.S., Dai D-F. TOR signaling pathway in cardiac aging and heart failure. Biomolecules. 2021;11:168. doi: 10.3390/biom11020168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang S., Long H., Hou L., Feng B., Ma Z., Wu Y., et al. The mitophagy pathway and its implications in human diseases. Signal Transduct Target Ther. 2023;8:304. doi: 10.1038/s41392-023-01503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Youle R.J., Narendra D.P. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Springer W., Kahle P.J. Regulation of PINK1-Parkin-mediated mitophagy. Autophagy. 2011;7:266–278. doi: 10.4161/auto.7.3.14348. [DOI] [PubMed] [Google Scholar]
- 55.Tolkovsky A.M. Mitophagy. Biochim Biophys Acta. 2009;1793(9):1508–1515. doi: 10.1016/j.bbamcr.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 56.Zorova L.D., Popkov V.A., Plotnikov E.Y., Silachev D.N., Pevzner I.B., Jankauskas S.S., et al. Mitochondrial membrane potential. Anal Biochem. 2018;552:50–59. doi: 10.1016/j.ab.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hasson S.A., Kane L.A., Yamano K., Huang C.H., Sliter D.A., Buehler E., et al. High-content genome-wide RNAi screens identify regulators of parkin upstream of mitophagy. Nature. 2013;504:291–295. doi: 10.1038/nature12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Narendra D.P., Jin S.M., Tanaka A., Suen D.F., Gautier C.A., Shen J., et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000298. e1000298–e1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Y., Dorn G.W. PINK1-phosphorylated mitofusin 2 is a parkin receptor for culling damaged mitochondria. Science (1979) 2013;340:471–475. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dodson M., Darley-Usmar V., Zhang J. Cellular metabolic and autophagic pathways: traffic control by redox signaling. Free Radic Biol Med. 2013;63:207–221. doi: 10.1016/j.freeradbiomed.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kang R., Zeh H.J., Lotze M.T., Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18:571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zachari M., Ganley I.G. The mammalian ULK1 complex and autophagy initiation. Essays Biochem. 2017;61:585–596. doi: 10.1042/EBC20170021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee Y., Lee H.Y., Hanna R.A., Gustafsson A.B., Chen M., Chen Z., et al. Mitophagy receptor FUNDC1 regulates mitochondrial dynamics and mitophagy. Am J Physiol Heart Circ Physiol. 2011;12:H1924–H1931. [Google Scholar]
- 64.Strappazzon F., Nazio F., Corrado M., Cianfanelli V., Romagnoli A., Fimia G.M., et al. AMBRA1 is able to induce mitophagy via LC3 binding, regardless of PARKIN and p62/SQSTM1. Cell Death Differ. 2015;22:517. doi: 10.1038/cdd.2014.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Strappazzon F., Nazio F., Corrado M., Cianfanelli V., Romagnoli A., Fimia G.M., et al. AMBRA1 is able to induce mitophagy via LC3 binding, regardless of PARKIN and p62/SQSTM1. Cell Death Differ. 2015;22:419–432. doi: 10.1038/cdd.2014.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ney P.A. Mitochondrial autophagy: origins, significance, and role of BNIP3 and NIX. Biochim Biophys Acta. 2015;1853:2775–2783. doi: 10.1016/j.bbamcr.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 67.Liu L., Feng D., Chen G., Chen M., Zheng Q., Song P., et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat Cell Biol. 2012;14:177–185. doi: 10.1038/ncb2422. [DOI] [PubMed] [Google Scholar]
- 68.Zheng P., Ma W., Gu Y., Wu H., Bian Z., Liu N., et al. High-fat diet causes mitochondrial damage and downregulation of mitofusin-2 and optic atrophy-1 in multiple organs. J Clin Biochem Nutr. 2023;73:61. doi: 10.3164/jcbn.22-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu H., Zang C., Yuan F., Ju C., Shang M., Ning J., et al. The role of FUNDC1 in mitophagy, mitochondrial dynamics and human diseases. Biochem Pharmacol. 2022;197 doi: 10.1016/j.bcp.2021.114891. [DOI] [PubMed] [Google Scholar]
- 70.Zhang J., Ney P.A. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ. 2009;16:939–946. doi: 10.1038/cdd.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Novak I., Kirkin V., McEwan D.G., Zhang J., Wild P., Rozenknop A., et al. Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 2010;11:45–51. doi: 10.1038/embor.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Twig G., Elorza A., Molina A.J.A., Mohamed H., Wikstrom J.D., Walzer G., et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kremer L.S., Bozhilova L.V., Rubalcava-Gracia D., Filograna R., Upadhyay M., Koolmeister C., et al. A role for BCL2L13 and autophagy in germline purifying selection of mtDNA. PLoS Genet. 2023;19 doi: 10.1371/journal.pgen.1010573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park S., Choi S.G., Yoo S.M., Son J.H., Jung Y.K. Choline dehydrogenase interacts with SQSTM1/p62 to recruit LC3 and stimulate mitophagy. Autophagy. 2014;10:1906–1920. doi: 10.4161/auto.32177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seabright A.P., Fine N.H.F., Barlow J.P., Lord S.O., Musa I., Gray A., et al. AMPK activation induces mitophagy and promotes mitochondrial fission while activating TBK1 in a PINK1-Parkin independent manner. FASEB J. 2020;34:6284. doi: 10.1096/fj.201903051R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saito T., Nah J., ichi O.S, Mukai R., Monden Y., Maejima Y., et al. An alternative mitophagy pathway mediated by Rab9 protects the heart against ischemia. J Clin Investig. 2019;129:802–819. doi: 10.1172/JCI122035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gelmetti V., De Rosa P., Torosantucci L., Marini E.S., Romagnoli A., Di Rienzo M., et al. PINK1 and BECN1 relocalize at mitochondria-associated membranes during mitophagy and promote ER-mitochondria tethering and autophagosome formation. Autophagy. 2017;13:654–669. doi: 10.1080/15548627.2016.1277309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alsayyah C., Ozturk O., Cavellini L., Belgareh-Touzé N., Cohen M.M. The regulation of mitochondrial homeostasis by the ubiquitin proteasome system. Biochim Biophys Acta (BBA)-Bioenerget. 2020;1861 doi: 10.1016/j.bbabio.2020.148302. [DOI] [PubMed] [Google Scholar]
- 79.Wong Y.C., Holzbaur E.L.F. Optineurin is an autophagy receptor for damaged mitochondria in parkin-mediated mitophagy that is disrupted by an ALS-linked mutation. Proc Natl Acad Sci USA. 2014;111:E4439–E4448. doi: 10.1073/pnas.1405752111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kubli D.A., Gustafsson Å.B. Unbreak my heart: targeting mitochondrial autophagy in diabetic cardiomyopathy. Antioxid Redox Signal. 2015;22:1527–1544. doi: 10.1089/ars.2015.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu X., Kobayashi S., Chen K., Timm D., Volden P., Huang Y., et al. Diminished autophagy limits cardiac injury in mouse models of type 1 diabetes. J Biol Chem. 2013;288:18077–18092. doi: 10.1074/jbc.M113.474650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu Y., Jiang T., Hua J., Xiong Z., Dai K., Chen H., et al. PINK1/Parkin-mediated mitophagy in cardiovascular disease: from pathogenesis to novel therapy. Int J Cardiol. 2022;361:61–69. doi: 10.1016/j.ijcard.2022.05.025. [DOI] [PubMed] [Google Scholar]
- 84.Apostolova N., Vezza T., Muntané J., Rocha M., Victor V.M. Mitochondrial dysfunction and mitophagy in type 2 diabetes: pathophysiology and therapeutic targets. Antioxid Redox Signal. 2023;39:4–6. doi: 10.1089/ars.2022.0016. [DOI] [PubMed] [Google Scholar]
- 85.Ma R.C.W., Tong P.C.Y. Epidemiology of type 2 diabetes. Textb Diabetes. 2024:55–74. [Google Scholar]
- 86.Caturano A., D'Angelo M., Mormone A., Russo V., Mollica M.P., Salvatore T., et al. Oxidative stress in type 2 diabetes: impacts from pathogenesis to lifestyle modifications. Curr Issues Mol Biol. 2023;45:6651–6666. doi: 10.3390/cimb45080420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Garg S.S., Gupta J. Polyol pathway and redox balance in diabetes. Pharmacol Res. 2022;182 doi: 10.1016/j.phrs.2022.106326. [DOI] [PubMed] [Google Scholar]
- 88.McLean F.H., Campbell F.M., Langston R.F., Sergi D., Resch C., Grant C., et al. A high-fat diet induces rapid changes in the mouse hypothalamic proteome. Nutr Metab (Lond) 2019;16:1–16. doi: 10.1186/s12986-019-0352-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sciarretta S., Zhai P., Shao D., Maejima Y., Robbins J., Volpe M., et al. Rheb is a critical regulator of autophagy during myocardial ischemia: pathophysiological implications in obesity and metabolic syndrome. Circulation. 2012;125:1134–1146. doi: 10.1161/CIRCULATIONAHA.111.078212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu X., Hua Y., Nair S., Zhang Y., Erratum Ren J. Akt2 knockout preserves cardiac function in high-fat diet-induced obesity by rescuing cardiac autophagosome maturation. J Mol Cell Biol. 2013;5:212. doi: 10.1093/jmcb/mjs055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Filardi T., Ghinassi B., Di Baldassarre A., Tanzilli G., Morano S., Lenzi A., et al. Cardiomyopathy associated with diabetes: the central role of the cardiomyocyte. Int J Mol Sci. 2019;20:3299. doi: 10.3390/ijms20133299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jia G., Hill M.A., Sowers J.R. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018;122:624–638. doi: 10.1161/CIRCRESAHA.117.311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Khan S., Ahmad S.S., Kamal M.A. Diabetic cardiomyopathy: from mechanism to management in a nutshell. Endocr Metab Immune Disord Drug Targets. 2021;21:268–281. doi: 10.2174/1871530320666200731174724. [DOI] [PubMed] [Google Scholar]
- 94.Lee W.S., Kim J. Diabetic cardiomyopathy: where we are and where we are going. Korean J Intern Med. 2017;32:404–421. doi: 10.3904/kjim.2016.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu Y., Liu H., Wang X. Cardioprotection of pharmacological postconditioning on myocardial ischemia/reperfusion injury. Life Sci. 2021;264 doi: 10.1016/j.lfs.2020.118628. [DOI] [PubMed] [Google Scholar]
- 96.Tombo N., Imam Aliagan A.D., Feng Y., Singh H., Bopassa J.C. Cardiac ischemia/reperfusion stress reduces inner mitochondrial membrane protein (mitofilin) levels during early reperfusion. Free Radic Biol Med. 2020;158:181–194. doi: 10.1016/j.freeradbiomed.2020.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang W., Ren H., Xu C., Zhu C., Wu H., Liu D., et al. Hypoxic mitophagy regulates mitochondrial quality and platelet activation and determines severity of I/R heart injury. Elife. 2016;5:e21407. doi: 10.7554/eLife.21407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Titus A.S., Sung E.-A., Zablocki D., Sadoshima J. Mitophagy for cardioprotection. Basic Res Cardiol. 2023;118:1–24. doi: 10.1007/s00395-023-01009-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Luan Y., Luan Y., Feng Q., Chen X., Ren K.D., Yang Y. Emerging role of mitophagy in the heart: therapeutic potentials to modulate mitophagy in cardiac diseases. Oxid Med Cell Longev. 2021;2021 doi: 10.1155/2021/3259963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xiong W., Ma Z., An D., Liu Z., Cai W., Bai Y., et al. Mitofusin 2 participates in mitophagy and mitochondrial fusion against angiotensin II-induced cardiomyocyte injury. Front Physiol. 2019;10:411. doi: 10.3389/fphys.2019.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shires S.E., Gustafsson Å.B. Mitophagy and heart failure. J Mol Med. 2015;93:253–262. doi: 10.1007/s00109-015-1254-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou H., He L., Xu G., Chen L. Mitophagy in cardiovascular disease. Clin Chim Acta. 2020;507:210–218. doi: 10.1016/j.cca.2020.04.033. [DOI] [PubMed] [Google Scholar]
- 103.Torp M., Vaage J., Stensløkken K. Mitochondria-derived damage-associated molecular patterns and inflammation in the ischemic-reperfused heart. Acta Physiol. 2023;237:e13920. doi: 10.1111/apha.13920. [DOI] [PubMed] [Google Scholar]
- 104.Oka T., Hikoso S., Yamaguchi O., Taneike M., Takeda T., Tamai T., et al. Erratum: mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;490:292. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lemmerman L.R., Das D., Higuita-Castro N., Mirmira R.G., Gallego-Perez D. Nanomedicine-based strategies for diabetes: diagnostics, monitoring, and treatment. Trends Endocrinol Metab. 2020;31:448–458. doi: 10.1016/j.tem.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bishnoi M., Deepika M.N, Jain A. In: Nanotechnology for biomedical applications. materials horizons: from nature to nanomaterials. Gopi S, Balakrishnan P, Mubarak NM, editors. Springer; Singapore: 2022. Biomedical applications of nano-biosensor. materials horizons: from nature to nanomaterials; pp. 219–246. [Google Scholar]
- 107.Ghosh A., Nag S., Gomes A., Gosavi A., Ghule G., Kundu A., et al. Applications of smart material sensors and soft electronics in healthcare wearables for better user compliance. Micromachines (Basel) 2022;14:121. doi: 10.3390/mi14010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Padma S., Chakraborty P., Mukherjee S. In: Next-generation nanobiosensor devices for point-of-care diagnostics. Dutta G, editor. Springer; Singapore: 2023. Nano-biosensors for diagnosing infectious and lifestyle-related disease of human: an update; pp. 79–103. [Google Scholar]
- 109.Salek-Maghsoudi A., Vakhshiteh F., Torabi R., Hassani S., Ganjali M.R., Norouzi P., et al. Recent advances in biosensor technology in assessment of early diabetes biomarkers. Biosens Bioelectron. 2018;99:122–135. doi: 10.1016/j.bios.2017.07.047. [DOI] [PubMed] [Google Scholar]
- 110.Liu Y., Zeng S., Ji W., Yao H., Lin L., Cui H., et al. Emerging theranostic nanomaterials in diabetes and its complications. Adv Sci. 2022;9 doi: 10.1002/advs.202102466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chetty R., Singh V.P., Madhusudhan A., Wilson R., Rodriguez-Nieves A. Functional smart nanomaterials and their theranostics approaches. Springer; 2024. Nanostructured electrodes as electrochemical biosensors for biomedical applications; pp. 241–261. [Google Scholar]
- 112.Sumitha M.S., Xavier T.S. Recent advances in electrochemical biosensors–A brief review. Hybrid Adv. 2023;2 [Google Scholar]
- 113.Szunerits S., Mishyn V., Grabowska I., Boukherroub R. Electrochemical cardiovascular platforms: current state of the art and beyond. Biosens Bioelectron. 2019;131:287–298. doi: 10.1016/j.bios.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 114.Ahmed I., Jiang N., Shao X., Elsherif M., Alam F., Salih A., et al. Recent advances in optical sensors for continuous glucose monitoring. Sens Diagn. 2022;1:1098–1125. [Google Scholar]
- 115.Qureshi A., Gurbuz Y., Niazi J.H. Biosensors for cardiac biomarkers detection: a review. Sens Actuators B Chem. 2012;171–172:62–76. [Google Scholar]
- 116.Wang S., Luo Y., Wen C., Zhao S., Zhang L. An autocatalytically-activatable hydrogen peroxide photoacoustic sensor for in situ visualization precise diagnosis and drug intervention tracing in diabetes syndrome. Biosens Bioelectron. 2023;222 doi: 10.1016/j.bios.2022.114964. [DOI] [PubMed] [Google Scholar]
- 117.Liang J., Yan K., Liu Y., Yao X., Guo F., Xue W., et al. ArGO-PAM-Fc/AuNPs nanosensing membrane in a light-addressable potentiometric biosensor for 1,5-anhydroglucitol determination. Microchem J. 2023;184 [Google Scholar]
- 118.Zhang R., Liu L., Li W., Luo X., Wu F. Luminescent carbon dots with excellent peroxidase mimicking property for fluorometric and colorimetric detection of glucose. Colloids Surf B Biointerfaces. 2023;222 doi: 10.1016/j.colsurfb.2023.113125. [DOI] [PubMed] [Google Scholar]
- 119.Goswami P.P., Deshpande T., Rotake D.R., Singh S.G. Near perfect classification of cardiac biomarker Troponin-I in human serum assisted by SnS2-CNT composite, explainable ML, and operating-voltage-selection-algorithm. Biosens Bioelectron. 2023;220 doi: 10.1016/j.bios.2022.114915. [DOI] [PubMed] [Google Scholar]
- 120.Hosseinniya S., Rezayan A.H., Ghasemi F., Malekmohamadi M., Taheri R.A., Hosseini M., et al. Fabrication and evaluation of optical nanobiosensor based on localized surface plasmon resonance (LSPR) of gold nanorod for detection of CRP. Anal Chim Acta. 2023;1237 doi: 10.1016/j.aca.2022.340580. [DOI] [PubMed] [Google Scholar]
- 121.Liu R., Zhang S., Wang T., Hu N., Ma X., Zhu L. Nanostructured NiCu LDHs/polypyrrole nanotubes composite for nonenzymatic glucose sensing in human serum. Electroanalysis. 2023;35(5) [Google Scholar]
- 122.Ramesh A., Ajith A., Gudipati N.S., Vanjari S.R.K., John S.A., Biju V., et al. Hybridization of Co3S4 and graphitic carbon nitride nanosheets for high-performance nonenzymatic sensing of H2O2. Biosensors (Basel) 2023;13:108. doi: 10.3390/bios13010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yan Q., Wu R., Chen H., Wang H., Nan W. Highly sensitive cholesterol biosensor based on electron mediator thionine and cubic-shaped Cu2O nanomaterials. Microchem J. 2023;185 [Google Scholar]
- 124.Kumar A., Gupta G.H., Singh G., More N., Keerthana M., Sharma A., et al. Ultrahigh sensitive graphene oxide/conducting polymer composite based biosensor for cholesterol and bilirubin detection. Biosens Bioelectron X. 2023;13 [Google Scholar]
- 125.Yola M.L., Atar N. Amperometric galectin-3 immunosensor-based gold nanoparticle-functionalized graphitic carbon nitride nanosheets and core-shell Ti-MOF@COFs composites. Nanoscale. 2020;12:19824–19832. doi: 10.1039/d0nr05614f. [DOI] [PubMed] [Google Scholar]
- 126.Chen M., Zhao L., Wu D., Tu S., Chen C., Guo H., et al. Highly sensitive sandwich-type immunosensor with enhanced electrocatalytic durian-shaped MoS2/AuPtPd nanoparticles for human growth differentiation factor-15 detection. Anal Chim Acta. 2022;1223 doi: 10.1016/j.aca.2022.340194. [DOI] [PubMed] [Google Scholar]
- 127.Ben Halima H., Bellagambi F.G., Hangouët M., Alcacer A., Pfeiffer N., Heuberger A., et al. A novel electrochemical strategy for NT-proBNP detection using IMFET for monitoring heart failure by saliva analysis. Talanta. 2023;251 doi: 10.1016/j.talanta.2022.123759. [DOI] [PubMed] [Google Scholar]
- 128.Mansoor S., Shahid S., Ashiq K., Alwadai N., Javed M., Iqbal S., et al. Controlled growth of nanocomposite thin layer based on Zn-Doped MgO nanoparticles through Sol-Gel technique for biosensor applications. Inorg Chem Commun. 2022;142 [Google Scholar]
- 129.Surya S.G., Majhi S.M., Lahcen A.A., Yuvaraja S., Chappanda K.N., Salama K.N., et al. A label-free aptasensor FET based on Au nanoparticle decorated Co3O4 nanorods and a SWCNT layer for detection of cardiac troponin T protein. J Mater Chem B. 2019;8:18–26. doi: 10.1039/c9tb01989h. [DOI] [PubMed] [Google Scholar]
- 130.Cheng Y., Xu H., Cao W., Gao W., Tang B. Cerium fluoride nanoparticles as a theranostic material for optical imaging of vulnerable atherosclerosis plaques. J Am Ceram Soc. 2023;106:2375–2383. [Google Scholar]
- 131.Dong X., Zhao G., Li X., Miao J.C., Fang J., Wei Q., et al. Electrochemiluminescence immunoassay for the N-terminal pro-B-type natriuretic peptide based on resonance energy transfer between a self-enhanced luminophore composed of silver nanocubes on gold nanoparticles and a metal-organic framework of type MIL-125. Microchim Acta. 2019;186:1–8. doi: 10.1007/s00604-019-3969-5. [DOI] [PubMed] [Google Scholar]
- 132.Liu W., Zhou X., Xu L., Zhu S., Yang S., Chen X., et al. Graphene quantum dot-functionalized three-dimensional ordered mesoporous ZnO for acetone detection toward diagnosis of diabetes. Nanoscale. 2019;11:11496–11504. doi: 10.1039/c9nr00942f. [DOI] [PubMed] [Google Scholar]
- 133.Kanwal A., Kanwar N., Bharati S., Srivastava P., Singh S.P., Amar S. Exploring new drug targets for type 2 diabetes: success, challenges and opportunities. Biomedicines. 2022;10:331. doi: 10.3390/biomedicines10020331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Longo M., Scappaticcio L., Cirillo P., Maio A., Carotenuto R., Maiorino M.I., et al. Glycemic control and the heart: the tale of diabetic cardiomyopathy continues. Biomolecules. 2022;12:272. doi: 10.3390/biom12020272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gilbert M.P., Pratley R.E. GLP-1 analogs and dpp-4 inhibitors in type 2 diabetes therapy: review of head-to-head clinical trials. Front Endocrinol (Lausanne) 2020;11:178. doi: 10.3389/fendo.2020.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kuate Defo A., Bakula V., Pisaturo A., Labos C., Wing S.S., Daskalopoulou S.S. Diabetes, antidiabetic medications and risk of dementia: a systematic umbrella review and meta-analysis. Diabetes Obes Metab. 2023;26:441–462. doi: 10.1111/dom.15331. [DOI] [PubMed] [Google Scholar]
- 137.Shyamaladevi B., Dash I., Badrachalam R., Krishnan M., Panneerselvam A., Undru S. An update on diagnosis and therapeutics for type-2 diabetes mellitus. Bioinformation. 2023;19:295. doi: 10.6026/97320630019295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Padhi S., Nayak A.K., Behera A. Type II diabetes mellitus: a review on recent drug based therapeutics. Biomed Pharmacother. 2020;131 doi: 10.1016/j.biopha.2020.110708. [DOI] [PubMed] [Google Scholar]
- 139.Beysel S., Unsal I.O., Kizilgul M., Caliskan M., Ucan B., Cakal E. The effects of metformin in type 1 diabetes mellitus. BMC Endocr Disord. 2018;18:1–6. doi: 10.1186/s12902-017-0228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chaudhury A., Duvoor C., Reddy Dendi V.S., Kraleti S., Chada A., Ravilla R., et al. Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management. Front Endocrinol (Lausanne) 2017;8:6. doi: 10.3389/fendo.2017.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yue Y., Meng K., Pu Y., Zhang X. Transforming growth factor beta (TGF-β) mediates cardiac fibrosis and induces diabetic cardiomyopathy. Diabetes Res Clin Pract. 2017;133:124–130. doi: 10.1016/j.diabres.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 142.Arad M., Waldman M., Abraham N.G., Hochhauser E. Therapeutic approaches to diabetic cardiomyopathy: targeting the antioxidant pathway. Prostagland Lipid Mediat. 2020;150 doi: 10.1016/j.prostaglandins.2020.106454. [DOI] [PubMed] [Google Scholar]
- 143.Murtaza G., Virk H.U.H., Khalid M., Lavie C.J., Ventura H., Mukherjee D., et al. Diabetic cardiomyopathy - A comprehensive updated review. Prog Cardiovasc Dis. 2019;62:315–326. doi: 10.1016/j.pcad.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 144.Borghetti G., Von Lewinski D., Eaton D.M., Sourij H., Houser S.R., Wallner M. Diabetic cardiomyopathy: current and future therapies. Beyond glycemic control. Front Physiol. 2018;9:1514. doi: 10.3389/fphys.2018.01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mazin I., Chernomordik F., Fefer P., Matetzky S., Beigel R. The impact of novel anti-diabetic medications on cv outcomes: a new therapeutic horizon for diabetic and non-diabetic cardiac patients. J Clin Med. 2022;11:1904. doi: 10.3390/jcm11071904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Soccio R.E., Chen E.R., Lazar M.A. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab. 2014;20:573–591. doi: 10.1016/j.cmet.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.He B.K., Ning Z.Q., Li Z.B., Shan S., Pan D.S., Ko B.C.B., et al. In vitro and in vivo characterizations of chiglitazar, a newly identified PPAR pan-agonist. PPAR Res. 2012;2012 doi: 10.1155/2012/546548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stein E., Bays H., Koren M., Bakker-Arkema R., Bisgaier C. Efficacy and safety of gemcabene as add-on to stable statin therapy in hypercholesterolemic patients. J Clin Lipidol. 2016;10:1212–1222. doi: 10.1016/j.jacl.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 149.Packer M., Anker S.D., Butler J., Filippatos G., Pocock S.J., Carson P., et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 150.Skelley J.W., Carter B.S., Roberts M.Z. Clinical potential of canagliflozin in cardiovascular risk reduction in patients with type 2 diabetes. Vasc Health Risk Manag. 2018;14:419–428. doi: 10.2147/VHRM.S168472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Guthrie R. Canagliflozin and cardiovascular and renal events in type 2 diabetes. Postgrad Med. 2018;130:149–153. doi: 10.1080/00325481.2018.1423852. [DOI] [PubMed] [Google Scholar]
- 152.Rachid O., Osman A., Abdi R., Haik Y. CTLA4-Ig (abatacept): a promising investigational drug for use in type 1 diabetes. Expert Opin Investig Drugs. 2020;29:221–236. doi: 10.1080/13543784.2020.1727885. [DOI] [PubMed] [Google Scholar]
- 153.Chavda V.P., Ajabiya J., Teli D., Bojarska J., Apostolopoulos V. Tirzepatide, a new era of dual-targeted treatment for diabetes and obesity: a mini-review. Molecules. 2022;27:4315. doi: 10.3390/molecules27134315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dvorakova M.C., Mistrova E., Paddenberg R., Kummer W., Slavikova J. Substance P receptor in the rat heart and regulation of its expression in long-term diabetes. Front Physiol. 2018;9:918. doi: 10.3389/fphys.2018.00918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gutch M., Joshi A., Kumar S., Agarwal A., Pahan R.K., Razi S.M. Gemigliptin: newer promising gliptin for type 2 diabetes mellitus. Indian J Endocrinol Metab. 2017;21:898–902. doi: 10.4103/ijem.IJEM_20_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tang Q., Pan W., Peng L. The efficacy and safety of evogliptin for type 2 diabetes mellitus: a systematic review and meta-analysis. Front Endocrinol (Lausanne) 2022;13 doi: 10.3389/fendo.2022.962385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bhatt D.L., Szarek M., Steg P.G., Cannon C.P., Leiter L.A., McGuire D.K., et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384:117–128. doi: 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
- 158.Allegretti A.S., Zhang W., Zhou W., Thurber T.K., Rigby S.P., Bowman-Stroud C., et al. Safety and effectiveness of bexagliflozin in patients with type 2 diabetes mellitus and stage 3a/3b CKD. Am J Kidney Dis. 2019;74:328–337. doi: 10.1053/j.ajkd.2019.03.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li X., Huang X., Bai C., Qin D., Cao S., Mei Q., et al. Efficacy and safety of teneligliptin in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol. 2018;9:449. doi: 10.3389/fphar.2018.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Arai H., Yamashita S., Yokote K., Araki E., Suganami H., Ishibashi S. Efficacy and safety of pemafibrate versus fenofibrate in patients with high triglyceride and low HDL cholesterol levels: a multicenter, placebo-controlled, double-blind, randomized trial. J Atheroscler Thromb. 2018;25:521–538. doi: 10.5551/jat.44412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jia W., Bai T., Zeng J., Niu Z., Fan D., Xu X., et al. Combined administration of metformin and atorvastatin attenuates diabetic cardiomyopathy by inhibiting inflammation, apoptosis, and oxidative stress in type 2 diabetic mice. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.634900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lombardo D., Kiselev M.A., Caccamo M.T. Smart nanoparticles for drug delivery application: development of versatile nanocarrier platforms in biotechnology and nanomedicine. J Nanomater. 2019;2019 [Google Scholar]
- 163.Chen G., Roy I., Yang C., Prasad P.N. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem Rev. 2016;116:2826–2885. doi: 10.1021/acs.chemrev.5b00148. [DOI] [PubMed] [Google Scholar]
- 164.Ul Haq M.N., Shah G.M., Gul A., Foudah A.I., Alqarni M.H., Yusufoglu H.S., et al. Biogenic synthesis of silver nanoparticles using phagnalon niveum and its in vivo anti-diabetic effect against alloxan-induced diabetic wistar rats. Nanomaterials. 2022;12:830. doi: 10.3390/nano12050830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Katsiki N., Purrello F., Tsioufis C., Mikhailidis D.P. Cardiovascular disease prevention strategies for type 2 diabetes mellitus. Expert Opin Pharmacother. 2017;18:1243–1260. doi: 10.1080/14656566.2017.1351946. [DOI] [PubMed] [Google Scholar]
- 166.Díaz-Pozo P., Canet F., Grirrane A., Lopez-Domenech S., Herance J.R., Apostolova N., et al. Gold nanoparticles supported on ceria nanoparticles modulate leukocyte–endothelium cell interactions and inflammation in type 2 diabetes. Antioxidants. 2022;11:2297. doi: 10.3390/antiox11112297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gao W., Liang L. Effect of polysaccharide sulfate-loaded poly(lactic-co-glycolic acid) nanoparticles on coronary microvascular dysfunction of diabetic cardiomyopathy. J Biomed Nanotechnol. 2022;18:446–452. doi: 10.1166/jbn.2022.3261. [DOI] [PubMed] [Google Scholar]
- 168.Dunlay S.M., Givertz M.M., Aguilar D., Allen L.A., Chan M., Desai A.S., et al. Type 2 diabetes mellitus and heart failure: a scientific statement from the American Heart Association and the Heart Failure Society of America: this statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation. 2019;140:e294–e324. doi: 10.1161/CIR.0000000000000691. [DOI] [PubMed] [Google Scholar]
- 169.Shan M., Feng N., Zhang L. Efficacy of heparinoid PSS in treating cardiovascular diseases and beyond—A review of 31 years clinical experiences in China. Prog Mol Biol Transl Sci. 2019;163:75–93. doi: 10.1016/bs.pmbts.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 170.Miragoli M., Ceriotti P., Iafisco M., Vacchiano M., Salvarani N., Alogna A., et al. Inhalation of peptide-loaded nanoparticles improves heart failure. Sci Transl Med. 2018;10:eaan6205. doi: 10.1126/scitranslmed.aan6205. [DOI] [PubMed] [Google Scholar]
- 171.El-Sayed A., Kamel M. Advances in nanomedical applications: diagnostic, therapeutic, immunization, and vaccine production. Environ Sci Pollut Res. 2020;27:19200–19213. doi: 10.1007/s11356-019-06459-2. [DOI] [PubMed] [Google Scholar]
- 172.Singh A.V., Varma M., Laux P., Choudhary S., Datusalia A.K., Gupta N., et al. Artificial intelligence and machine learning disciplines with the potential to improve the nanotoxicology and nanomedicine fields: a comprehensive review. Arch Toxicol. 2023;97:963–979. doi: 10.1007/s00204-023-03471-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xie C., Liao J., Zhang N., Sun Y., Li Y., Xiong L., et al. Advanced nano drug delivery systems for neuroprotection against ischemic stroke. Chin Chem Lett. 2023;35(2) [Google Scholar]
- 174.Lee Y.K., Diaz B., Deroose M., Lee S.X., Belvedere S., Accili D., et al. FOXO1 inhibition synergizes with FGF21 to normalize glucose control in diabetic mice. Mol Metab. 2021;49 doi: 10.1016/j.molmet.2021.101187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Langlet F., Haeusler R.A., Lindén D., Ericson E., Norris T., Johansson A., et al. Selective inhibition of FOXO1 activator/repressor balance modulates hepatic glucose handling. Cell. 2017;171:824–835. doi: 10.1016/j.cell.2017.09.045. .e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xu K., Liu X.F., Ke Z.Q., Yao Q., Guo S., Liu C. Resveratrol modulates apoptosis and autophagy induced by high glucose and palmitate in cardiac cells. Cell Physiol Biochem. 2018;46:2031–2040. doi: 10.1159/000489442. [DOI] [PubMed] [Google Scholar]
- 177.Oka M., Kato N. Aldose reductase inhibitors. J Enzyme Inhib. 2001;16:465–473. doi: 10.1080/14756360127568. [DOI] [PubMed] [Google Scholar]
- 178.Jannapureddy S. Aldose reductase: an emerging target for development of interventions for diabetic cardiovascular complications. Front Endocrinol (Lausanne) 2021;12 doi: 10.3389/fendo.2021.636267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Thakur S., Gupta S.K., Ali V., Singh P., Verma M. Aldose Reductase: a cause and a potential target for the treatment of diabetic complications. Arch Pharm Res. 2021;44:655–667. doi: 10.1007/s12272-021-01343-5. [DOI] [PubMed] [Google Scholar]
- 180.Raish M., Ahmad A., Bin Jardan Y.A., Shahid M., Alkharfy K.M., Ahad A., et al. Sinapic acid ameliorates cardiac dysfunction and cardiomyopathy by modulating NF-κB and Nrf2/HO-1 signaling pathways in streptozocin induced diabetic rats. Biomed Pharmacother. 2022;145 doi: 10.1016/j.biopha.2021.112412. [DOI] [PubMed] [Google Scholar]
- 181.Lai R.C., Arslan F., Lee M.M., Sze N.S.K., Choo A., Chen T.S., et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 182.Chen L., Wang Y., Pan Y., Zhang L., Shen C., Qin G., et al. Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem Biophys Res Commun. 2013;431:566–571. doi: 10.1016/j.bbrc.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yu B., Kim H.W., Gong M., Wang J., Millard R.W., Wang Y., et al. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int J Cardiol. 2015;182:349–360. doi: 10.1016/j.ijcard.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li H., Zhu H., Ge T., Wang Z., Zhang C. Mesenchymal stem cell-based therapy for diabetes mellitus: enhancement strategies and future perspectives. Stem Cell Rev Rep. 2021;17:1552–1569. doi: 10.1007/s12015-021-10139-5. [DOI] [PubMed] [Google Scholar]
- 185.Chen M.T., Zhao Y.T., Zhou L.Y., Li M., Zhang Q., Han Q., et al. Exosomes derived from human umbilical cord mesenchymal stem cells enhance insulin sensitivity in insulin resistant human adipocytes. Curr Med Sci. 2021;41:87–93. doi: 10.1007/s11596-021-2323-4. [DOI] [PubMed] [Google Scholar]
- 186.Nagy G., Szekely T.E., Somogyi A., Herold M., Herold Z. New therapeutic approaches for type 1 diabetes: disease-modifying therapies. World J Diabetes. 2022;13:835–850. doi: 10.4239/wjd.v13.i10.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhang M., Zhu N.W., Ma W.C., Chen M.J., Zheng L. Combined treatment with ultrasound-targeted microbubble destruction technique and NM-aFGF-loaded PEG-nanoliposomes protects against diabetic cardiomyopathy-induced oxidative stress by activating the AKT/GSK-3β1/Nrf-2 pathway. Drug Deliv. 2020;27:938–952. doi: 10.1080/10717544.2020.1785052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chandrakala V., Aruna V., Angajala G. Review on metal nanoparticles as nanocarriers: current challenges and perspectives in drug delivery systems. Emergent Mater. 2022;5:1593–1615. doi: 10.1007/s42247-021-00335-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Fang H., Huang L., Lv F., Hu B., Liu H., Huang Z., et al. Dual-responsive targeted atherosclerosis therapy through a multi-effective nanoplatform with anti-inflammatory, lipid-regulating and autophagy. Chem Eng J. 2023;454 [Google Scholar]
- 190.Gao W., Sun Y., Cai M., Zhao Y., Cao W., Liu Z., et al. Copper sulfide nanoparticles as a photothermal switch for TRPV1 signaling to attenuate atherosclerosis. Nat Commun. 2018;9:231. doi: 10.1038/s41467-017-02657-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Montiel Schneider M.G., Lassalle V.L. Magnetic iron oxide nanoparticles as novel and efficient tools for atherosclerosis diagnosis. Biomed Pharmacother. 2017;93:1098–1115. doi: 10.1016/j.biopha.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 192.Duan J., Du J., Jin R., Zhu W., Liu L., Yang L., et al. Iron oxide nanoparticles promote vascular endothelial cells survival from oxidative stress by enhancement of autophagy. Regen Biomater. 2019;6:221–229. doi: 10.1093/rb/rbz024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Aroca A., Gotor C. Hydrogen sulfide: a key role in autophagy regulation from plants to mammalians. Antioxidants. 2022;11:327. doi: 10.3390/antiox11020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kang B., Li W., Xi W., Yi Y., Ciren Y., Shen H., et al. Hydrogen sulfide protects cardiomyocytes against apoptosis in ischemia/reperfusion through MiR-1-regulated histone deacetylase 4 pathway. Cell Physiol Biochem. 2017;41:10–21. doi: 10.1159/000455816. [DOI] [PubMed] [Google Scholar]
- 195.Mo W., Shen J., Huang X., Zhang Y., Zhang Z. Acute myocardial injury following hydrogen sulfide poisoning. Toxicol Ind Health. 2020;36:750–758. doi: 10.1177/0748233720945184. [DOI] [PubMed] [Google Scholar]
- 196.Rumbeiha W., Whitley E., Anantharam P., Kim D.S., Kanthasamy A. Acute hydrogen sulfide–induced neuropathology and neurological sequelae: challenges for translational neuroprotective research. Ann N Y Acad Sci. 2016;1378:5–16. doi: 10.1111/nyas.13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hanaoka K., Sasakura K., Suwanai Y., Toma-Fukai S., Shimamoto K., Takano Y., et al. Discovery and mechanistic characterization of selective inhibitors of H2S-producing Enzyme: 3-Mercaptopyruvate. Sci Rep. 2017;7:40227. doi: 10.1038/srep40227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Petrosino M., Zuhra K., Kopec J., Hutchin A., Szabo C., Majtan T. H2S biogenesis by cystathionine beta-synthase: mechanism of inhibition by aminooxyacetic acid and unexpected role of serine. Cell Mol Life Sci. 2022;79:438. doi: 10.1007/s00018-022-04479-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wang Q., Wang P. Angiotensin 1 peptide conjugated CdSe/ZnS quantum dots induce cardiac-specific hydrogen sulfide production to mitigate myocardial ischemia-reperfusion injury. Res Sq. 2021 [Google Scholar]
- 200.Dalli J., Montero-Melendez T., McArthur S., Perretti M. Annexin A1 N-terminal derived peptide Ac2-26 exerts chemokinetic effects on human neutrophils. Front Pharmacol. 2012;3:28. doi: 10.3389/fphar.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Liu C., Chen L., Ma Y., Hu K., Wu P., Pan L., et al. Pulmonary circulation-mediated heart targeting for the prevention of heart failure by inhalation of intrinsically bioactive nanoparticles. Theranostics. 2021;11:8550–8569. doi: 10.7150/thno.61875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nitta S.K., Numata K. Biopolymer-based nanoparticles for drug/gene delivery and tissue engineering. Int J Mol Sci. 2013;14:1629–1654. doi: 10.3390/ijms14011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhang M., Zhu J., Qin X., Zhou M., Zhang X., Gao Y., et al. Cardioprotection of Tetrahedral DNA nanostructures in myocardial ischemia-reperfusion injury. ACS Appl Mater Interfaces. 2019;11:30631–30639. doi: 10.1021/acsami.9b10645. [DOI] [PubMed] [Google Scholar]
- 204.Ray P., Haideri N., Haque I., Mohammed O., Chakraborty S., Banerjee S., et al. The impact of nanoparticles on the immune system: a gray zone of nanomedicine. J Immunol Sci. 2021;5:19–33. [Google Scholar]
- 205.Liu Y., Hardie J., Zhang X., Rotello V.M. Effects of engineered nanoparticles on the innate immune system. Semin Immunol. 2017;34:25–32. doi: 10.1016/j.smim.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sidhu A.K., Verma N., Kaushal P. Role of Biogenic capping agents in the synthesis of metallic nanoparticles and evaluation of their therapeutic potential. Front Nanotechnol. 2022;3:105. [Google Scholar]
- 207.Chen P., Wang H., He M., Chen B., Yang B., Hu B. Size-dependent cytotoxicity study of ZnO nanoparticles in HepG2 cells. Ecotoxicol Environ Saf. 2019;171:337–346. doi: 10.1016/j.ecoenv.2018.12.096. [DOI] [PubMed] [Google Scholar]
- 208.Poon W., Zhang Y.N., Ouyang B., Kingston B.R., Wu J.L.Y., Wilhelm S., et al. Elimination pathways of nanoparticles. ACS Nano. 2019;13:5785–5798. doi: 10.1021/acsnano.9b01383. [DOI] [PubMed] [Google Scholar]
- 209.Zhang N., Xiong G., Liu Z. Toxicity of metal-based nanoparticles: challenges in the nano era. Front Bioeng Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.1001572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kawata K., Osawa M., Okabe S. In vitro toxicity of silver nanoparticles at noncytotoxic doses to HepG2 human hepatoma cells. Environ Sci Technol. 2009;43:6046–6051. doi: 10.1021/es900754q. [DOI] [PubMed] [Google Scholar]
- 211.Yue L., Zhao W., Wang D., Meng M., Zheng Y., Li Y., et al. Silver nanoparticles inhibit beige fat function and promote adiposity. Mol Metab. 2019;22:1–11. doi: 10.1016/j.molmet.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Singh A.M., Zhang L., Avery J., Yin A., Du Y., Wang H., et al. Human beige adipocytes for drug discovery and cell therapy in metabolic diseases. Nat Commun. 2020;11:2758. doi: 10.1038/s41467-020-16340-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lizcano F. The beige adipocyte as a therapy for metabolic diseases. Int J Mol Sci. 2019;20:5058. doi: 10.3390/ijms20205058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Guertl B., Noehammer C., Hoefler G. Metabolic cardiomyopathies. Int J Exp Pathol. 2000;81:349–372. doi: 10.1046/j.1365-2613.2000.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.McCubrey J.A., LaHair M.M., Franklin R.A. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal. 2006;8:1775–1789. doi: 10.1089/ars.2006.8.1775. [DOI] [PubMed] [Google Scholar]
- 216.Van Haute D, JM Berlin. Challenges in realizing selectivity for nanoparticle biodistribution and clearance: lessons from gold nanoparticles. Ther Deliv. 2017;8:763–774. doi: 10.4155/tde-2017-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hu X., Cook S., Wang P., Hwang H. In vitro evaluation of cytotoxicity of engineered metal oxide nanoparticles. Sci Total Environ. 2009;407:3070–3072. doi: 10.1016/j.scitotenv.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 218.Guan R., Kang T., Lu F., Zhang Z., Shen H., Liu M. Cytotoxicity, oxidative stress, and genotoxicity in human hepatocyte and embryonic kidney cells exposed to ZnO nanoparticles. Nanoscale Res Lett. 2012;7:1–7. doi: 10.1186/1556-276X-7-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ahmed F., Husain Q., Ansari M.O., Shadab G. Antidiabetic and oxidative stress assessment of bio-enzymatically synthesized zinc oxide nanoformulation on streptozotocin-induced hyperglycemic mice. Appl Nanosci. 2020;10:879–893. [Google Scholar]
- 220.Gogitidze Joy N., Hedrington M.S., Briscoe V.J., Tate D.B., Ertl A.C., Davis S.N. Effects of acute hypoglycemia on inflammatory and pro-atherothrombotic biomarkers in individuals with type 1 diabetes and healthy individuals. Diabetes Care. 2010;33:1529–1535. doi: 10.2337/dc09-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sanità G., Carrese B., Lamberti A. Nanoparticle surface functionalization: how to improve biocompatibility and cellular internalization. Front Mol Biosci. 2020;7 doi: 10.3389/fmolb.2020.587012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Adão R., Stoddart P.R., Peter K. Avalanching nanoparticles bring new light to cardiovascular imaging. Cardiovasc Res. 2021;117:E60–E63. doi: 10.1093/cvr/cvab092. [DOI] [PubMed] [Google Scholar]
- 223.Rydosz A. In: Nanosensors for Smart Cities. Han B., Tomer V., Nguyen T., et al., editors. Elsevier; 2020. Nanosensors for exhaled breath monitoring as a possible tool for noninvasive diabetes detection; pp. 467–481. [Google Scholar]
- 224.Liu Y., Zhang D., Qu Y., Tang F., Wang H., Ding A., et al. Advances in small-molecule fluorescent pH probes for monitoring mitophagy. Chem Biomed Imaging. 2023;2:81–164. [Google Scholar]
- 225.Ke Y., Castro C., Choi J.H. Structural DNA nanotechnology: artificial nanostructures for biomedical research. Annu Rev Biomed Eng. 2018;20:375–401. doi: 10.1146/annurev-bioeng-062117-120904. [DOI] [PubMed] [Google Scholar]
- 226.Tanzina H.S., Chowdhury E. Recent progress in delivery of therapeutic and imaging agents utilizing organic-inorganic hybrid nanoparticles. Curr Drug Deliv. 2017;14:485–496. doi: 10.2174/1567201814666171120114034. [DOI] [PubMed] [Google Scholar]
- 227.Fan C., Joshi J., Li F., Xu B., Khan M., Yang J., et al. Nanoparticle-mediated drug delivery for treatment of ischemic heart disease. Front Bioeng Biotechnol. 2020;8:687. doi: 10.3389/fbioe.2020.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hathaway Q.A., Roth S.M., Pinti M.V., Sprando D.C., Kunovac A., Durr A.J., et al. Machine-learning to stratify diabetic patients using novel cardiac biomarkers and integrative genomics. Cardiovasc Diabetol. 2019;18:1–16. doi: 10.1186/s12933-019-0879-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Chen Z.Z., Gerszten R.E. Metabolomics and proteomics in type 2 diabetes. Circ Res. 2020;126:1613–1627. doi: 10.1161/CIRCRESAHA.120.315898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Tayanloo-Beik A., Roudsari P.P., Rezaei-Tavirani M., Biglar M., Tabatabaei-Malazy O., Arjmand B., et al. Diabetes and heart failure: multi-omics approaches. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.705424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sun Y., Gao H.Y., Fan Z.Y., He Y., Yan Y.X. Metabolomics signatures in type 2 diabetes: a systematic review and integrative analysis. J Clin Endocrinol Metab. 2020;105:1000–1008. doi: 10.1210/clinem/dgz240. [DOI] [PubMed] [Google Scholar]
- 232.Nomura A., Noguchi M., Kometani M., Furukawa K., Yoneda T. Artificial intelligence in current diabetes management and prediction. Curr Diab Rep. 2021;21:61. doi: 10.1007/s11892-021-01423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ellahham S. Artificial intelligence: the future for diabetes care. Am J Med. 2020;133:895–900. doi: 10.1016/j.amjmed.2020.03.033. [DOI] [PubMed] [Google Scholar]
- 234.Chopra H., Bibi S., Mishra A.K., Tirth V., Yerramsetty S.V., Murali S.V., et al. Nanomaterials: a promising therapeutic approach for cardiovascular diseases. J Nanomater. 2022;2022:1–25. [Google Scholar]
- 235.Liu P., Chen G., Zhang J. A review of liposomes as a drug delivery system: current status of approved products, regulatory environments, and future perspectives. Molecules. 2022;27:1372. doi: 10.3390/molecules27041372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Marinheiro D., Martel F., Ferreira B.J.M.L., Daniel-da-Silva A.L. Silica-based nanomaterials for diabetes mellitus treatment. Bioengineering. 2023;10:40. doi: 10.3390/bioengineering10010040. [DOI] [PMC free article] [PubMed] [Google Scholar]