Abstract
In the first study, an in vitro culture system was developed to investigate the effects of carnosine on macrophage proinflammatory cytokine response using an established chicken macrophage cell line (CMC), gut integrity using a chicken intestinal epithelial cell line (IEC), muscle differentiation in quail muscle cells (QMCs) and primary chicken embryonic muscle cells (PMCs), and direct anti-parasitic effect against Eimeria maxima sporozoites. Cells to be tested were seeded in 24-well plates and treated with carnosine at 4 different concentrations (0.1, 1.0, and 10.0 µg). After 18 h of incubation, cells were harvested to measure gene expression of proinflammatory cytokines in CMC, tight junction (TJ) proteins in IECs, and muscle cell growth markers in QMCs and PMCs. In vivo trials were conducted to investigate the effect of dietary carnosine on disease parameters in broiler chickens challenged with E. maxima. One hundred and twenty male broiler chickens (0-day-old) were allocated into 4 treatment groups: 1) basal diet without infection (NC), 2) basal diet with E. maxima infection (PC), 3) carnosine at 10.0 mg/kg feed with PC (HCS), and 4) carnosine at 1.0 mg/kg feed with PC (LCS). All groups except NC were orally infected with E. maxima on d 14. Jejunal samples were collected for lesion scoring and jejunum gut tissues were used for transcriptomic analysis of cytokines and TJ proteins. In vitro, carnosine treatment significantly decreased IL-1β gene expression in CMC following LPS stimulation. In vivo feeding studies showed that dietary carnosine increased BW and ADG of chickens in E. maxima-infected groups and reduced the jejunal lesion score and fecal oocyst shedding in HCS group. Jejunal IL-1β, IL-8, and IFN-γ expression were suppressed in the HCS group compared to PC. The expression levels of claudin-1 and occludin in IECs were also increased in HCS following carnosine treatment. In conclusion, these findings highlight the beneficial effects of dietary carnosine supplementation on intestinal immune responses and gut barrier function in broiler chickens exposed to E. maxima infection.
Key words: carnosine, feed additive, gut health, gut metabolite, in vitro test
INTRODUCTION
In the postantibiotic era, developing novel nonantibiotic feed additives has become increasingly important for controlling poultry diseases and improving the intestinal health and growth performance of commercial poultry (Granstad et al., 2020; Lee et al., 2021). There are many nonantibiotic feed additives that have been shown to enhance growth performance, gut health, and mitigation of disease responses in chickens in postantibiotic era. Examples of antibiotic alternative feed additives that showed beneficial effects in poultry production are probiotics, prebiotics, organic acids, enzymes, phytochemicals, antioxidants, vitamins, and minerals (Shehata et al., 2022; Abd El-Hack et al., 2022; Ajibola et al., 2023). The goal of enhancing intestinal health and decreasing the negative effects of diseases through the use of these functional additives in their feed is to maintain gut health and improving animal growth performance by optimizing host immunity, reducing gut damage and reducing the presence of pathogens and anti-nutritional factors that compromise animal health (Mahfuz et al., 2021; Lee et al., 2022a; Wickramasuriya et al., 2022). Since postantibiotic era, there have been many advances in formulating novel feed additives that reduces negative effects of diseases in commercial poultry production. Advances in the development, understanding their modes of action and the commercialization of novel feed additives will facilitate successful application of antibiotic alternative feed additives to promote poultry and animal health.
Recent technical advances in genomics have contributed to a better understanding of the composition of the intestinal microbiome, the beneficial roles of diverse microbiome species, and the identification of potential biomarkers associated with gut health and growth performance in chickens (He et al., 2021; Segura-Wang et al., 2021). While genomics can provide information on changes in gene expression, metabolomics reveals the actual alterations in metabolic pathways that exert beneficial effects on gut function (Lee and Hase, 2014). The establishment of a causal relationship between microbiota, metabolites, and the host physiology through the use of genomics and metabolomics will facilitate the development of microbiota-targeted precision nutrition for chickens and animals, thereby improving their intestinal health and growth (Tebani and Bekri, 2019; Levatte et al., 2022; Livingstone et al., 2022). Recently, omics technologies have been gaining attention as various strategies aimed at exploring and manipulating the intestinal ecosystem in order to maintain homeostasis as a healthy intestine even in challenging circumstances such as unbalanced nutrition or disease challenges (Brugaletta et al., 2022; Arenas-Gómez et al., 2023; Urgessa and Woldesemayat, 2023).
In our previous study, we have discovered beneficial effects of dietary supplementation of young broiler chickens with 2 strains of Bacillus subtilis 1,781 and 747 that enhanced intestinal immune responses, epithelial barrier integrity, and growth performance in chickens infected with Eimeria maxima. (Gadde et al., 2017; Park et al., 2020a). A subsequent metabolomics study on the ileum of chickens fed with combination of Bacillus subtilis 1,781 and 747 (Park et al., 2020b) led to the identification of total of 158 amino acid (AA)-related metabolites, with 33 increased and 158 decreased levels. Of the 33 amino acid-related metabolites increased compared to the control group, carnosine showed the highest relative increase, with a fold change of 4.93 (unpublished data). Carnosine, a dipeptide consisting of β-alanine and histidine, is widely distributed in various tissues of animals, including chickens (Boldyrev et al., 2013; Kopec et al., 2020). The presence of carnosine in many chicken tissues, such as the muscles, liver, kidneys, heart, intestine, and brain, suggests their important role in cellular functions (Boldyrev et al., 2013; Wu, 2020). In the chicken intestine, carnosine is found in lower concentrations compared to other tissues. Nevertheless, its presence in the intestine suggests potential roles in intestinal health and functions (Wu, 2020; Jukić et al., 2021). Studies have indicated that carnosine exhibits anti-inflammatory and antioxidant properties, improves the integrity of the intestinal barrier, and modulates host immune system. These functions of carnosine may be attributed to its ability to strengthen the tight junctions (TJs) between cells in the intestinal lining and its modulation of host local immune responses in the intestine (Mahmood et al., 2007; Davison et al., 2016).
Therefore, we hypothesized that dietary carnosine supplementation in chickens infected with Eimeria spp could alter the inflammatory response, permeability, and nutrient transport in the brush border of the intestine, which could lead to changes in growth.
To verify the hypothesis, the study aimed to:
-
1.
Assess the effects of carnosine on the immune response and gut integrity in vitro using chicken cells, its anti-parasite effect, and its influence on muscle cell differentiation.
-
2.
Evaluate the impact of dietary carnosine on growth, immunity, gut health, and nutrient absorption in broiler chickens infected with E. maxima.
These studies will provide valuable insights into the mechanisms underlying the beneficial physiological changes associated with the gut metabolites generated from the dietary direct-fed microbial feed additives.
MATERIALS AND METHODS
Experiment 1: In Vitro Study
Culture of Epithelial Chicken Cell Line and Chicken Macrophage Cell Line
Epithelial chicken cells (IECs, MM-CHiC clone, 8E11, MicroMol GmbH, Germany) and chicken macrophage cells (CMCs, chicken macrophage-like line, HD11, MicroMol GmbH) were seeded at a concentration of 2 × 105 cells/mL in 24-well plates and cultured in the Dulbecco's modified Eagle medium (DMEM)/F-12 (Hyclone, Logan, UT) containing 10% heat-inactivated fetal bovine serum (FBS, Hyclone) and 1% antibiotic solution [penicillin (10,000 unit/mL) and streptomycin (10 mg/mL), Gibco, Grand Island, NY]. Cells were incubated at 41°C in a humidified atmosphere containing 5% CO2 for 24 h to allow for cell adhesion. After 24 h, lipopolysaccharide (LPS, Sigma-Aldrich, St. Louis, MO) was added to each well at a concentration of 1.0 µg/mL along with carnosine (Sigma-Aldrich) at concentrations of 0.0, 0.1, 1.0, and 10.0 µg/mL. After an additional incubation of 18 h, cells were collected using lysis buffer (Qiagen, Valencia, CA) and 2-mercaptoethanol (Sigma-Aldrich). RNA was extracted from the IEC and the CMC using the RNeasy Isolation Kit (Qiagen) on a QIAcube (Qiagen), and gene expression was analyzed by quantitative real-time polymerase chain reaction (qRT-PCR). All experiments were performed independently at least 3 times.
Anticoccidial Assay Against E. maxima
The direct effect of carnosine on Eimeria parasites was evaluated using in vitro sporozoite viability assay as previously described by Kim et al. (2017). Briefly, fresh sporulated oocysts were added in a 2 mL microcentrifuge tube and disrupted with glass beads (0.5 mm) in the Mini-Bead beater (BioSpec Products, Bartlesville, OK) for 10 s. Released sporocysts were rinsed in chilled Hanks' balanced salt solution (HBSS, Hyclone) and incubated with 10 mL of excystation media (0.25% trypsin and 0.014 M taurocholic acid, pH 7.4) at 41°C for 1 h to release sporozoites. Sporozoites (2.5 × 105) were seeded in each well of a 96-well plate. Chicken NK-lysin (cNK-lysin, Genscript, Piscataway, NJ) at concentrations of 1.0, 10, and 100 µg/mL were used as positive controls. Carnosine was tested at 3 different concentrations: low (0.1 µg/mL), medium (1.0 µg/mL), and high (10 µg/mL). Freshly prepared live sporozoites were treated with carnosine and incubated at 41°C for 3 h. A fluorescent dye (AO/PI staining solution, Nexcelom Bioscience LLC, Lawrence, MA) was added to each well in a 1:1 ratio, and the number of live sporozoites was quantified using a Cellometer (Nexcelom Bioscience). All experiments were performed independently at least 3 times.
Quail Muscle Cell Culture
Quail muscle cell (QMCs) (2 × 105/mL) were seeded in 24-well plates according to previously methods (Shin et al., 2015). Cells were cultured in Medium 199 (Hyclone) containing 10% FBS and 1% antibiotic solution until cells reached 70% confluence. The media in 12 wells were replaced by Medium 199 containing 0.5% FBS and 1% antibiotic solution to induce cell differentiation, while the media in the remaining 12 wells were replaced with basic Medium 199 containing 10% FBS and 1% antibiotic solution to maintain cell proliferation. Carnosine was added to each well at concentrations of 0.0, 0.1, 1.0, and 10.0 µg/mL. After incubation at 41°C in a humidified atmosphere containing 5% CO2 for 18 h, cells were harvested using lysis buffer and 2-mercaptoethanol. RNA was extracted from QMCs using the RNeasy Isolation Kit on a QIAcube and gene expression was analyzed by qRT-PCR. All experiments were performed independently at least 3 times.
Primary Chicken Embryonic Muscle Cell Culture
Eggs for the embryonic muscle cell culture were obtained from Moyer's hatchery (Quakertown, PA). The culture protocol of primary chicken embryonic muscle cells (PMCs) was modified from method described by Hassan et al. (2014). Briefly, eggs were incubated at 41°C and 70% humidity. The pectoralis major region of 13-day-old embryos was extracted, minced, and digested with 0.05% trypsin-EDTA (Sigma-Aldrich) at 37°C for 20 min. PMCs were washed 2–3 times with HBSS and seeded at a concentration of 2 × 105 cells/mL in 24-well plates. Cells were maintained in DMEM (Hyclone) containing 10% FBS and 1% antibiotic solution until they reached 70% confluence. The media in 12 wells were replaced with DMEM containing 2% FBS and 1% antibiotic solution to induce cell differentiation, while the media in the remaining 12 wells were replaced with DMEM containing 10% FBS and 1% antibiotic solution to maintain cell proliferation. Carnosine was added to each well at concentrations of 0.0, 0.1, 1.0, and 10.0 µg/mL. After incubation at 41°C in a humidified atmosphere containing 5% CO2 for 18 h, cells were harvested using lysis buffer and 2-mercaptoethanol. RNA was extracted from PMCs using the RNeasy Isolation Kit on a QIAcube and eluted in 30 μL RNase-free water. Gene expression was analyzed by qRT-PCR. All experiments were performed independently at least 3 times.
Reverse Transcription From in Vitro Samples
The quantity of RNA was assessed using the NanoDrop (ND-1000) spectrophotometer (NanoDrop Products, Wilmington, DE) based on absorbance at 260 nm. RNA purity was evaluated by calculating the OD260/OD280 ratio. Total RNA (1 µg) was reverse-transcribed to cDNA using the QuantiTect reverse transcription kit (Qiagen). Briefly, the RNA samples were incubated with genomic DNA wipeout buffer (Qiagen) at 42°C for 2 min to remove any genomic DNA contamination. Reverse transcription (RT) was performed by adding Quantiscript Reverse Transcriptase, Quantiscript RT buffer, and RT primer mix to genomic DNA-depleted samples. The reaction was carried out in a thermal cycler (Mastercycler EP Gradient S; Eppendorf, Hauppauge, NY) with cycling conditions of 42°C for 30 min followed by reverse transcriptase inactivation at 95°C for 3 min. The cDNA samples were aliquots and stored at -20°C.
Analysis of Cytokines, Tight Junction Proteins, and Markers of Muscle Cell Growth by qRT-PCR
TJ protein expression (occludin, ZO-1, and MUC-2) was analyzed by qRT-PCR using RNA samples extracted from IECs. Levels of proinflammatory cytokines (IL-1β, IL-6, and IL-8) levels were measured in CMCs using extracted RNA samples. Proliferation and differentiation markers of muscle cells (Pax7 and MyoG) were determined using RNA samples obtained from QMCs and PMCs. qRT-PCR was performed using the Agilent Mx3000 P QPCR System (Agilent Technologies, Santa Clara, CA) and the Brilliant SYBR Green qRT-PCR Master Mix (Stratagene, La Jolla, CA). Oligonucleotide primer sequences and product size used for qRT-PCR are listed in Table 1. A melting curve was obtained at the end of each run to verify the presence of a single amplification product without primer dimers. Standard curves were generated using serial, 5-fold dilutions of cDNA. The fold changes in transcript levels were normalized to glyceraldehyde-3-phosphate dehydrogenase and are relative to the transcript expression in unstimulated control group (normalized to 1) using the comparative ΔΔ Ct method as previously described by Kim et al. (2014).
Table 1.
Ingredient composition of basal diet (as-fed basis, %, unless otherwise indicated).
| Ingredients (%) | Basal diet |
|---|---|
| Corn | 55.78 |
| Soybean meal | 37.03 |
| Soybean oil | 2.97 |
| Dicalcium phosphate | 1.80 |
| Calcium carbonate | 1.51 |
| Salt | 0.38 |
| Poultry Vit Mix1 | 0.22 |
| Poultry Mineral Mix2 | 0.15 |
| DL-Methionine | 0.10 |
| Choline-chloride, 60% | 0.06 |
| Total | 100.00 |
| Calculated values (%) | |
| CP, % | 24.00 |
| Ca, % | 1.20 |
| AP, % | 0.51 |
| Lys, % | 1.40 |
| Met, % | 0.49 |
| Cys + Met, % | 0.80 |
| ME, Mcal/kg | 3.5 |
Vitamin mixture provided the following nutrients per kg of diet: vitamin A, 2,000 IU; vitamin D3, 22 IU; vitamin E, 16 mg; vitamin K, 0.1 mg; vitamin B1, 3.4 mg; vitamin B2, 1.8 mg; vitamin B6, 6.4 mg; vitamin B12, 0.013 mg; biotin, 0.17 mg; pantothenic acid, 8.7 mg; folic acid, 0.8 mg; niacin, 23.8 mg.
Mineral mixture provided the following nutrients per kg of diet: Fe, 400 mg; Zn, 220 mg; Mn, 180 mg; Co, 1.3 mg; Cu, 21 mg; Se, 0.2 mg. CP = crude protein, AP = available phosphorus.
Experiment 2: In Vivo Study
This animal experiment was approved by the Beltsville Agricultural Research Center Institutional Animal Care and Use Committee (# 19–018). The experimental design is outlined in Figure 1.
Figure 1.
Schematic outline of the experimental design in experiment 2.
Chickens and Experimental Design
A total of 120 newly hatched male broiler chickens (Ross 708) were purchased from Longenecker's hatchery (Elizabethtown, PA) at 0 d of age. The day after arrival at the Beltsville ARS facility, chickens, which initially weighed 36.6 ± 0.7 g as an average body weight (BW), were allocated to 4 dietary treatments in a randomized complete block design with initial BW (heavy and light) as block. Dietary treatments included a corn- and soybean meal-based basal diet for noninfected chickens (NC, Table 2), a basal diet for E. maxima infected chickens (PC), PC supplemented with carnosine at 10.0 (HCS) and 1.0 mg/kg feed (LCS). Crystalline carnosine was purchased from Sigma-Aldrich. Each treatment group was allocated to 6 wire-bottom cages with 5 chickens per cage (0.65 × 0.75 m2). The chickens had ad libitum access to water and feed throughout the experimental period. A schematic outline of the experimental design is shown in Figure 1.
Table 2.
Oligonucleotide primer sequences for qRT-PCR.
| Type | Target gene | Primer sequence (5´-3´) | PCR product size (kb) |
|---|---|---|---|
| Reference | GAPDH | F-GGTGGTGCTAAGCGTGTTAT | 264 |
| R-ACCTCTGCCATCTCTCCACA | |||
| Proinflammatory | IL-1β | F-TGGGCATCAAGGGCTACA | 244 |
| R-TCGGGTTGGTTGGTGATG | |||
| IL-6 | F-CAAGGTGACGGAGGAGGAC | 254 | |
| R-TGGCGAGGAGGGATTTCT | |||
| IL-8 | F-GGCTTGCTAGGGGAAATGA | 200 | |
| R-AGCTGACTCTGACTAGGAAACTGT | |||
| TNFSF15 | F-CCTGAGTATTCCAGCAACGCA | 292 | |
| R-ATCCACCAGCTTGATGTCACTAAC | |||
| Th | IFN-γ | F-AGCTGACGGTGGACCTATTATT | 259 |
| R-GGCTTTGCGCTGGATTC | |||
| IL-10 | F-CGGGAGCTGAGGGTGAA | 272 | |
| R-GTGAAGAAGCGGTGACAGC | |||
| TJ proteins | Claudin-1 | F-CCTGATCACCCTCTTGGGAG | 145 |
| R-GCTGCACTCACTCATTGGCT | |||
| Claudin-2 | F-CCTGCTCACCCTCATTGGAG | 145 | |
| R-GCTGAACTCACTCTTGGGCT | |||
| JAM-2 | F-AGCCTCAAATGGGATTGGATT | 59 | |
| R-CATCAACTTGCATTCGCTTCA | |||
| Occludin | F-GAGCCCAGACTACCAAAGCAA | 68 | |
| R-GCTTGATGTGGAAGAGCTTGTTG | |||
| ZO-1 | F-CCGCAGTCGTTCACGATCT | 63 | |
| R-GGAGAATGTCTGGAATGGTCTGA | |||
| ZO-2 | F-ATCCAAGAAGGCACCTCAGC | 100 | |
| R-CATCCTCCCGAACAATGC | |||
| Muscle cell | MyoG | F-TGACCCTGTGCCCTGAAAGC | 178 |
| R-TCGTTCACCTTCTTCAGCCTCC | |||
| Pax7 | F-AAGGCCAAGCACAGCATAGA | 108 | |
| R-GCGCTGCTTCCTCTTCAAAG | |||
| Nutrient transports | B0AT | F-GGGTTTTGTGTTGGCTTAGGAA | 60 |
| R-TCCATGGCTCTGGCAGAGAT | |||
| B0+AT | F-CAGTAGTGAATTCTCTGAGTGTGAAGCT | 88 | |
| R-GCAATGATTGCCACAACTACCA | |||
| CAT1 | F-CCAAGCACGCTGATAAAG | 75 | |
| R-TACTCACAATAGGAAGAAGGG | |||
| EAAT | F-TGCTGCTTTGGATTCCAGTGT | 79 | |
| R-AGCAATGACTGTAGTGCAGAAGTAATATATG | |||
| GLUT1 | F-CTTTGTCAACCGCTTTGG | 65 | |
| R-TGTGCCCCGGAGCTTCT | |||
| GLUT2 | F-TCATTGTAGCTGAGCTGTT | 68 | |
| R-CGAAGACAACGAACACATAC | |||
| GLUT5 | F-TTGCTGGCTTTGGGTTGTG | 60 | |
| R-GGAGGTTGAGGGCCAAAGTC | |||
| LAT1 | F-GATTGCAACGGGTGATGTGA | 70 | |
| R-CCCCACACCCACTTTTGTTT | |||
| LAT2 | F-TCAGCTTCAGTTACTGGTT | 68 | |
| R-GCACAACCACGAGAAATAC | |||
| SGLT | F-GCCGTGGCCAGGGCTTA | 71 | |
| R-CAATAACCTGATCTGTGCACCAGT |
Th = T helper cells, TJ = tight junction, AhR = Aryl Hydrocarbon Receptor, B0AT = Na+-dependent amino acid transporter, B0+AT = Na+-independent amino acid transporter, CAT1 = cationic amino acid transporter, CYP1A4 = chicken cytochrome P-450 enzyme, CYP1A5 = chicken cytochrome P-450 enzyme, EAAT = excitatory amino acid transporter, GLUT1 = glucose transporter 1, GLUT2 = glucose transporter 2, GLUT5 = glucose transporter 5, LAT1 = L-type amino acid transporter 1, LAT2 = Na+-dependent neutral/cationic amino acid transporter, MyoG = Myogenin, Pax7= Paired Box 7, SGLT = sodium glucose transporter.
Determination of Body Weight
BW of chickens was measured at d 0, 7, 14, 20, and 22 to calculate average daily gain (ADG). Dead chickens were removed and weighed to adjust growth data.
Oral Infection With E. maxima
All chickens, except those in the NC group, were infected by oral gavage with E. maxima (1.0 × 104 sporulated oocysts/chicken, Beltsville strain 41A) on d 14 using previously described methods (Park et al., 2020a). The ratios of sporulated oocysts were 87.5 ± 2.1 %. The purity of the E. maxima infection was confirmed by DNA genotyping (Haug et al., 2007).
Collection of Intestinal Samples
One chicken with an average BW from each cage was euthanized by cervical dislocation on d 20, and their intestines were removed for further analysis. A small section (2 cm) of the distal jejunum without contents was collected aseptically from each intestine and stored in RNAlater (Invitrogen, Carlsbad, CA) at -20°C until further analysis.
Jejunal Lesion Scoring
Gut lesion scoring was performed on a 15-cm long distal jejunum sample on d 20. The intestinal lesion score was scored on a discrete ordinal scale of 0 (none) to 4 (high) by 4 independent observers in a blinded manner according to previously described methods (Lillehoj et al., 2016).
Fecal Oocyst Shedding
Fecal samples were collected from d 20 to 22 (6 to 8 d postinfection: dpi) and the number of oocysts was counted using the McMaster chamber according to previously described protocols (Park et al., 2022b) using the following formula:
Isolation of RNA and Reverse Transcription From Jejunal Samples
Total RNA was isolated from jejunum samples stored in RNAlater according to the manufacturer's recommendations. Approximately 50 mg of the jejunal tissue was homogenized in 1 mL of TRIzol (Invitrogen) using a hand-held homogenizer (TissueRuptor; Qiagen). Chloroform was added to the homogenized sample and the samples were centrifuged at 12,000 × g for 15 min at 4°C for separate phase. RNA in the colorless upper aqueous phase was precipitated using 100% isopropanol (Sigma-Aldrich). The RNA pellet was washed with 75% ethanol (Sigma-Aldrich), air-dried, and resuspended in RNase-free water (Invitrogen). The quantity of RNA was assessed using a NanoDrop (ND-1000) spectrophotometer (NanoDrop Products, Wilmington, DE) based on absorbance at 260 nm. RNA purity was evaluated by calculating the OD260/OD280 ratio. Total RNA (1 µg) was reverse-transcribed to cDNA using the QuantiTect reverse transcription kit (Qiagen). Briefly, RNA samples were incubated with genomic DNA wipeout buffer at 42°C for 2 min to remove any genomic DNA contamination. Reverse transcription (RT) was performed by adding Quantiscript Reverse Transcriptase, Quantiscript RT buffer, and RT primer mix to the genomic DNA-depleted samples. The reaction was carried out in a thermal cycler (Mastercycler EP Gradient S; Eppendorf, Hauppauge, NY) with cycling conditions of 42°C for 30 min followed by reverse transcriptase inactivation at 95°C for 3 min. cDNA samples were aliquots and stored at -20°C.
Gene Expression Analysis by qRT-PCR From the Extracted RNA
The oligonucleotide primer sequences used for qRT-PCR are listed in Table 1. Expression of various cytokines and intestinal TJ proteins was evaluated in the jejunum, including cytokines (IL-1β, IL-6, IL-8, IL-10, IFN-γ, and TNFSF15), TJ proteins (claudin-1, claudin-2, JAM-2, occludin, ZO-1, and ZO-2), and nutrient transporters (B0AT, B0+AT, CAT1, EAAT, GLUT1, GLUT2, GLUT5, LAT1 LAT2, and SGLT). Glyceraldehyde-3-phosphate dehydrogenase was used as the reference gene. Amplification and detection were performed using the Stratagene Mx3000P qPCR system (Agilent Technologies Inc.) and RT2 SYBR Green qPCR master mix (Qiagen). Each sample was analyzed in triplicate, and nonspecific primer amplification was assessed using no-template controls. Standard curves were generated using log10 diluted RNA standards and the transcript levels were normalized to those of glyceraldehyde-3-phosphate dehydrogenase using the Q-gene program (Kim et al., 2017).
Statistical Analysis
In vitro data for each response were analyzed using a PROC GLM in SAS version 9.4 (SAS Inc., Cary, NC). P-value less than 0.05 was considered significant. Data were accepted when skewness and kurtosis ranged in values at ± 3 and ± 7, respectively. In vivo data were analyzed using a mixed model (PROC MIXED) in SAS with each cage considered as an experimental unit. Results are presented as least squares mean values with pooled standard error of the mean. Probability values less than 0.05 were considered significantly different. When the overall effect was significant, mean values were compared pairwise using the PDIFF option.
RESULTS
Experiment 1
Effect of Carnosine on Tight Junction Proteins and Proinflammatory Cytokines
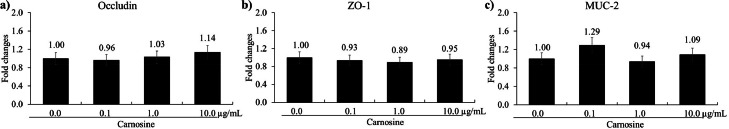
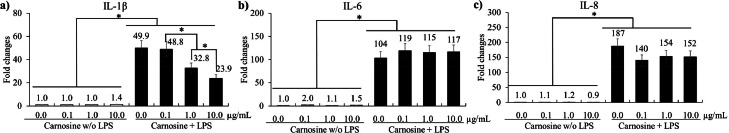
Carnosine administration did not affect occludin (Figure 2A), ZO-1 (Figure 2B), and MUC-2 (Figure 2C) levels in chicken IECs. LPS stimulation significantly increased (P < 0.05) IL-1β (Figure 3A, 1 to 49.9-fold), IL-6 (Figure 3B, 1 to 104-fold), and IL-8 (Figure 3C, 1 to 187-fold) levels in CMCs compared to groups without LPS. Carnosine decreased (P < 0.05) the expression of IL-1β at 1.0 (49.9 to 32.8-fold) and 10.0 (49.9 to 23.9-fold) μg/mL in CMCs compared to noncarnosine treated LPS group whereas carnosine administration did not change the levels of IL-6 and IL-8 in LPS groups. Carnosine was observed not to be involved in the regulation of TJ proteins in IECs, but it was involved in the inflammatory response that occurred in CMCs.
Figure 2.
mRNA expression level of tight junction proteins and mucin within cultured chicken epithelial cells by carnosine. Each bar represents the mean ± SEM (n = 3). Transcript levels of the tight junction proteins and mucin were measured using quantitative RT-PCR and normalized to GAPDH transcript levels.
Figure 3.
mRNA expression level of proinflammatory cytokines in chicken macrophages by LPS and carnosine. Each bar represents the mean ± SEM (n = 3). Transcript levels of the cytokines were measured using quantitative RT-PCR and normalized to GAPDH transcript levels. P-value less than 0.05 (*) was considered to be significant. LPS = lipopolysaccharide.
Effect of Carnosine on Proliferation and Differentiation of Quail Muscle Cells and Primary Chicken Embryonic Muscle Cells
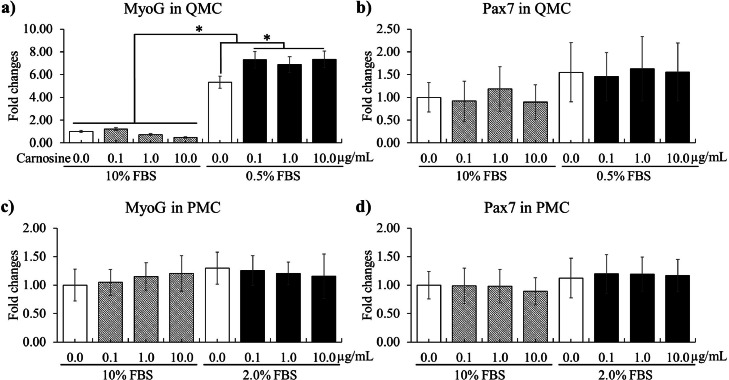
Reducing FBS concentration from 10 to 0.5% significantly increased (P < 0.05, 1 to 5.3-fold) the MyoG (Figure 4A) gene expression and carnosine administration regardless of the concentrations increased (P < 0.05, 5.3 to average 7.2-fold in carnosine groups) MyoG levels compared to 0.5% FBS group without carnosine. However, neither 0.5% FBS nor carnosine administrations affect Pax7 (Figure 4B) levels in QMCs. In PMCs, reducing FBS concentration to 2% and administering carnosine did not changed (P > 0.05) MyoG (Figure 4C) and Pax7 (Figure 4B) levels. These results suggested that carnosine affects QMCs in differentiation phase after proliferation.
Figure 4.
Proliferation and differentiation of quail muscle cells (QMC, a and b) and primary chicken embryonic muscle cells (PMC, c and d) by FBS concentration and carnosine. Each bar represents the mean ± SEM (n = 3). Transcript levels of the MyoG and Pax7 were measured using quantitative RT-PCR and normalized to GAPDH transcript levels. P-value less than 0.05 (*) was significant.
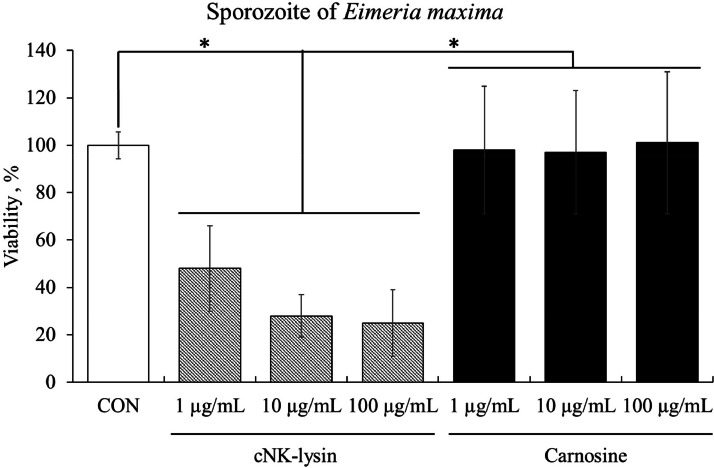
Carnosine on Anti-Coccidial Activity Against Sporozoites of E. maxima
As a positive control, we used cNK-lysin which showed a dose-dependently killing of (P < 0.05) E. maxima sporozoites by 48% to 25% compared to the CON group, whereas there was no difference between carnosine groups compared to the CON group (Figure 5). In this observation, it was found that carnosine does not have the ability to directly eliminate sporozoites of E. maxima.
Figure 5.
Anticoccidial effect of carnosine on sporozoites of E. maxima. Each bar represents the mean ± SEM (n = 3). The amount of sporozoite in CON group was 2.5 × 105 sporozoites/mL. P-value less than 0.05 (*) was significant. cNK-lysin as positive control showed a reduction of E. maxima sporozoites dose-dependently however carnosine did not appear to be directly associated with an anticoccidial effect.
Experiment 2
Dietary Effect of Carnosine on Chicken Growth Following Coccidiosis Infection
The initial BW did not differ significantly (P = 0.998) among the experimental groups (Table 3). Before infection (until d 14), dietary carnosine supplementation did not affect (P > 0.05) BW of chickens compared to the control group. E. maxima infection (PC) significantly decreased (P < 0.001) the BW of chicken at 6 dpi (860–743 g) and 8 dpi (1,017–763 g) compared to NC. HCS (P = 0.002, 763–870 g) and LCS (P = 0.007, 763 to 857 g) increased BW of chickens at 8 dpi compared to NC. Similarly, ADG of chickens fed diets supplemented with carnosine did not differ significantly (P > 0.05) from that of the control group. E. maxima-infected chickens (PC) showed lower ADG during 0 to 6 dpi (P < 0.001, 66.5–47.7 g) and 6 to 8 dpi (P < 0.001, 78.5–10.4 g) compared to NC. HCS (P < 0.001, 10.4–48.1 g) and LCS (P < 0.001, 10.4–56.3 g) increased ADG of infected chickens (PC) at 6 to 8 dpi. During the entire infection period (0–8 dpi), HCS (P < 0.0001, 38.2–50.3 g) and LCS (P < 0.001, 38.2–48.1 g) increased ADG of chickens compared to PC, which decreased due to E. maxima infection. Growth results indicate that dietary carnosine supplementation at both concentrations facilitated the recovery process following E. maxima infection and enhanced ADG.
Table 3.
Growth of chicken fed a diet supplemented with carnosine during infection with Eimeria maxima.
| Treatment | NC | PC | HCS | LCS | SEM | P-value |
|---|---|---|---|---|---|---|
| BW, g | ||||||
| Initial | 36.5 | 36.7 | 36.5 | 36.6 | 0.7 | 0.998 |
| D 7 | 157 | 159 | 159 | 154 | 2.8 | 0.538 |
| D 14 (0 dpi) | 461 | 457 | 469 | 459 | 7.9 | 0.725 |
| D 20 (6 dpi) | 860a | 743b | 774b | 735b | 18 | <.001 |
| D 22 (8 dpi) | 1,017a | 763c | 870b | 857b | 23 | <.001 |
| ADG, g | ||||||
| D 0 to 7 | 20.0 | 20.4 | 20.3 | 19.5 | 0.4 | 0.245 |
| D 7 to 14 | 43.5 | 41.9 | 44.3 | 43.4 | 0.8 | 0.222 |
| D 0 to 141 | 32.7 | 32.2 | 33.3 | 32.5 | 0.6 | 0.591 |
| D 14 to 20 | 66.5a | 47.7b | 50.9b | 46.2b | 1.8 | <.001 |
| D 14 to 222 | 69.4a | 38.2c | 50.3b | 48.1b | 2.0 | <.001 |
NC = basal diet, PC = basal diet for E. maxima-infected chickens, HCS = carnosine at 10.0 mg/kg feed, LCS = carnosine at 1.0 mg/kg feed, SEM = standard error of the mean
before infection
after infection, ADG = average daily gain, BW = body weight, D = day, dpi = days postinfection, all chickens except NC were infected by oral gavage at d 14 with 1.0 × 104 sporulated oocysts/chicken of E. maxima.
Means within the same row with different superscripts differ significantly (P < 0.05).
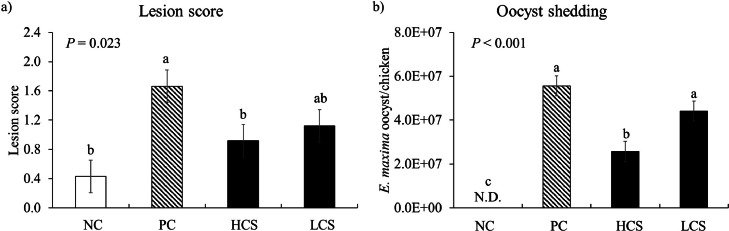
Intestinal Lesion Scores and Fecal Oocyst Shedding
E. maxima infection (PC) significantly increased (P = 0.004) jejunal lesion score (0.4–1.7) compared to NC (Figure 6A). HCS significantly decreased (P = 0.042) jejunal lesion score (1.7–0.9) compared to PC. Infection (PC) of chickens with E. maxima significantly increased (P < 0.001) oocyst numbers (0 to 5.6 × 107 oocyst/chicken) in feces compared to NC for 6 to 8 d postinfection whereas HCS significantly decreased (P = 0.004) the number of oocyst (5.6 × 107 to 2.6 × 107 oocyst/chicken) compared to PC (Figure 6B). There was no significant difference between PC and LCS groups. Ten mg of carnosine/kg feeding reduced the intestinal lesion score of the jejunum, and consistently, decreased number of fecal oocysts.
Figure 6.
Lesion score and oocyst shedding of chickens fed diet supplemented with carnosine during infection with E. maxima. NC = basal diet, PC = basal diet for infected chickens, HCS = carnosine at 10.0 mg/kg feed, LCS = carnosine at 1.0 mg/kg feed, N.D. = not detected. All chickens except NC were infected by oral gavage on d 14 with 1.0 × 104 oocysts/chicken of E. maxima. a,bBars with no common letter differ significantly (P < 0.05). Each bar represents the mean ± SEM (n = 6). The lesion score was collected from distal jejunal tissue on d 20 (6 d postinfection: dpi) and fecal sample was collected from 6 to 8 dpi to calculate the oocyst shedding.
Proinflammatory and Th Cytokines
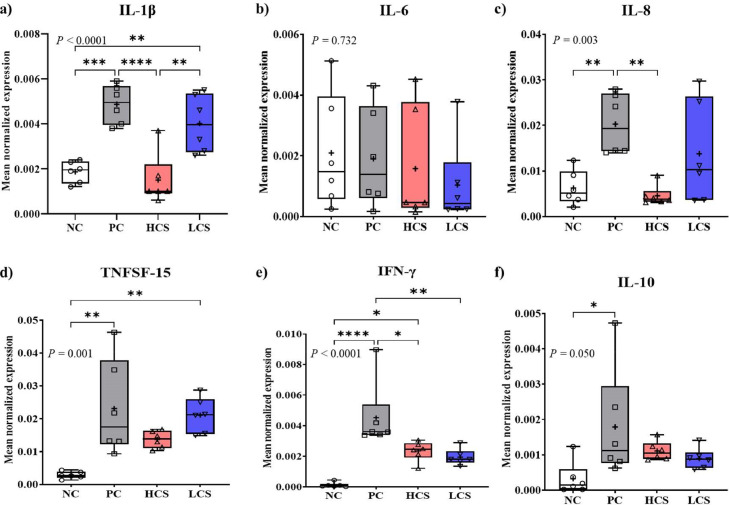
E. maxima infection (PC) significantly increased (P < 0.003) most cytokine levels [0.0019 to 0.0049 in IL-1β (Figure 7A), 0.006–0.02 in IL-8 (Figure 7C), 0.003 to 0.023 in TNFSF-15 (Figure 7D), 0.0001 to 0.0045 in IFN-γ (Figure 7E), and 0.0003 to 0.0018 in IL-10 (Figure 7F)] except IL-6 (Figure 6B), in the distal jejunum compared to NC, while HCS significantly decreased (P < 0.05) the expression levels of IL-1β (0.0049–0.0015), IL-8 (0.02 to 0.005), and IFN-γ (0.0045–0.0023) compared to PC. LCS significantly decreased (P < 0.01, 0.0045–0.0019) IFN-γ levels compared to PC. Jejunal IL-6 levels were not significantly changed (P > 0.05) by E. maxima infection or dietary carnosine supplementation at 6 d postinfection. Dietary carnosine supplementation did not significantly affect (P > 0.05) TNFSF-15 and IL-10 levels at 6 d postinfection which was increased by infection. Therefore, E. maxima infection induced an inflammatory response by increasing all measured cytokines levels except for IL-6, and dietary carnosine supplementation decreased the level of inflammatory response by suppressing the gene expression of IL-1β, IL-8, and IFN-γ, which were increased due to E. maxima infection.
Figure 7.
Gene expression of proinflammatory (a, b, c, and d) and T helper cell (e and f) cytokines in jejunum of chickens fed diets supplemented with carnosine during infection with E. maxima. Each box plot represented (from top to bottom) the maximum, upper quartile, medium, median, lower quartile, and minimum value. NC = basal diet, PC = basal diet for infected chickens, HCS = carnosine at 10.0 mg/kg feed, LCS = carnosine at 1.0 mg/kg feed. All chickens, except for NC were infected by oral gavage on d 14 with 1.0 × 104 sporulated oocysts/chicken of E. maxima. P < 0.05 (*), P < 0.01 (**), and P < 0.001 (***) were significant. The data (n = 6) were collected on d 20 (6 d postinfection). Transcript levels of the cytokines were measured using quantitative RT-PCR and normalized to GAPDH transcript levels.
Tight Junction Proteins
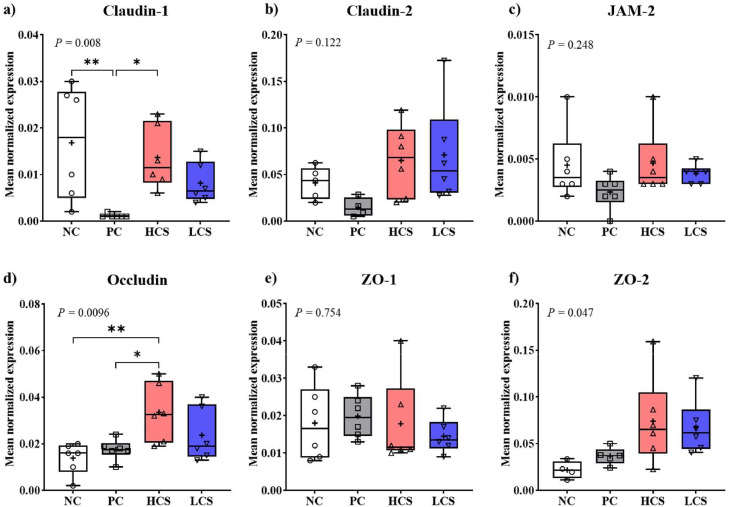
E. maxima-infection (PC) significantly decreased (P < 0.01, 0.017–0.001) the claudin-1 (Figure 8A) gene expression and HCS significantly increased (P < 0.05, 0.001–0.014) the claudin-1 levels compared to PC. Claudin-2 (Figure 8B) and JAM-2 (Figure 8C) levels showed a similar pattern to claudin-1 but were not significant (P > 0.05). Other TJ proteins [occludin (Figure 8D), ZO-1 (Figure 8E), and ZO-2 (Figure 8F)] were not significantly affected (P > 0.05) by E. maxima infection. HCS significantly increased (P < 0.05) the occludin levels compared to NC (0.014–0.034) and PC (0.018–0.034) (Figure 8D). For ZO-2 (Figure 8F), the P-value for all treated groups was significant (P = 0.047), but there was no significant between individual groups even though HCS (NC: 0.022–0.074, PC: 0.037–0.074) and LCS (NC: 0.022 to 0.068, PC:0.037–0.068) had higher ZO-2 levels compared to NC and PC. In this study, among the TJ proteins measured, only claudin was affected by E. maxima infection, and carnosine supplementation upregulated the level of jejunal claudin-1 and occludin in E. maxima-infected chickens, thereby enhancing intestinal permeability.
Figure 8.
Gene expression of tight junction proteins in jejunum of chickens fed diets supplemented with carnosine during infection with E. maxima. Each box plot represented (from top to bottom) the maximum, upper quartile, medium, median, lower quartile, and minimum value. NC = basal diet, PC = basal diet for infected chickens, HCS = carnosine at 10.0 mg/kg feed, LCS = carnosine at 1.0 mg/kg feed. All chickens, except for NC were infected by oral gavage on d 14 with 1.0 × 104 sporulated oocysts/chicken of E. maxima. P < 0.05 (*) and P < 0.01 (**) were significant. The data (n = 6) were collected on d 20 (6 d postinfection). Transcript levels of the cytokines were measured using quantitative RT-PCR and normalized to GAPDH transcript levels.
Nutrient Transporters
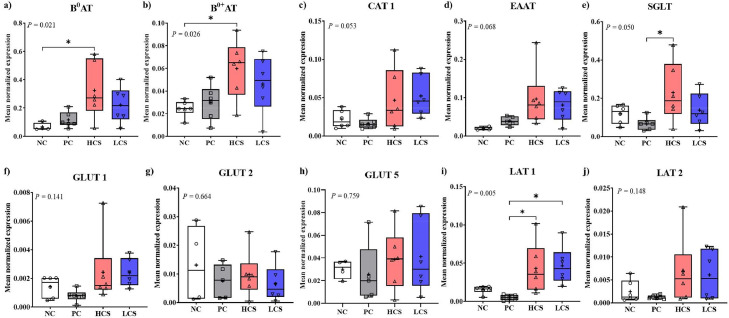
E. maxima infection (PC) did not significantly change (P > 0.05) nutrient transporter levels, although levels of CAT1 (Figure 9C, 0.022–0.016), SGLT (Figure 9E, 0.12–0.07), GLUT1 (Figure 9F, 0.0014–0.0008), GLUT5 (Figure 9H, 0.03–0.026), LAT1 (Figure 9I, 0.015–0.005), and LAT2 (Figure 9J, 0.0025–0.0012) were numerically decreased (P > 0.05). HCS significantly increased (P < 0.05) gene expression levels of B0AT (Figure 9A, 0.07–0.32) and B0+AT (Figure 9B, 0.025–0.06) in the jejunum of chickens compared to NC. SGLT (Figure 9E, 0.07–0.23) and LAT1 (Figure 9I, 0.005–0.043) levels were also elevated (P < 0.05) in HCS compared to PC. LCS increased (P < 0.05, 0.005–0.047) the gene expression level of LAT1 more than that of PC (Figure 9I). E. maxima infection or carnosine supplementation showed no significant effect on EAAT (Figure 9D) and GLUT2 (Figure 9G). In these results, it was not very consistent, but due to the jejunum damaged by E. mixaima infection, a tendency was observed for the gene expression level of some nutrient transporters to decrease. In this regard, carnosine supplementation may influence the damaged transporters by facilitating their recovery.
Figure 9.
Gene expression of nutrient transporters in jejunum of chickens fed diets supplemented with carnosine during infection with E. maxima. Each box plot represented (from top to bottom) the maximum, upper quartile, medium, median, lower quartile, and minimum value. NC = basal diet, PC = basal diet for infected chickens, HCS = carnosine at 10.0 mg/kg feed, LCS = carnosine at 1.0 mg/kg feed. All chickens, except for NC were infected by oral gavage on d 14 with 1.0 × 104 sporulated oocysts/chicken of E. maxima. P < 0.05 (*) was significant. The data (n = 6) were collected on d 20 (6 d postinfection). Transcript levels of the cytokines were measured using quantitative RT-PCR and normalized to GAPDH transcript levels.
DISCUSSION
Carnosine treatment in vitro of IECs did not change any tight junction proteins and mucin levels even though other studies in human and mouse cell lines showed the improved TJ proteins. Zn-carnosine administration to Caco-2 and HT29, human epithelial cell lines, increased total occludin expression and reduced phosphorylated tyrosine claudin, phosphorylated tyrosine occludin, and phosphorylated serine occludin under heat stress thereby enhancing the TJ formation (Davison et al., 2016). However, most of the studies were conducted using either human or mouse cell lines and there has been very limited information studying the tight junction proteins of the IECs (CHiC 8E11) in chickens.
As expected, in the current study, LPS stimulation of CMCs induced a significant inflammatory response characterized by an upregulation of cytokine release. Specifically, the levels of IL-1β, IL-6, and IL-8 were markedly increased by 50-, 104-, and 187-folds, respectively. The administration of carnosine led to a dose-dependent suppression of the inflammatory response, resulting in the reductions of IL-1β levels up to 35% at the 1.0 µg and 52% at the 10.0 µg. These findings are consistent with previous research demonstrating the anti-inflammatory effects of carnosine in different cell lines (Fleisher-Berkovich et al., 2009; Fresta et al., 2020; Kubota et al., 2020). A causal relationship between cytokine release and carnosine has already been documented well through various studies (Sun et al., 2021). Nevertheless, there was no information of cytokine release related to the carnosine treatment of CMCs (HD11). Based on the cytokine results of the current study, carnosine in CMCs may mitigate LPS-induced inflammatory response by suppressing the cytokine secretion.
As for the other physiological functions of carnosine, carnosine has been shown to affect muscle contraction, prevent the accumulation of oxidative metabolism by-products and act as an intracellular proton buffer maintaining the muscle acid-base balance. The current study evaluated carnosine capability to affect muscle growth using QMCs and PMCs. MyoG (known as myogenin) is a gene that plays a critical role in muscle cell differentiation and is required for muscle fiber differentiation. MyoG regulates the expression of several genes that are essential for muscle formation, such as genes encoding contractile proteins, and it also contributes to the maturation of muscle fibers (Cornelison and Wold, 1997; Halevy et al., 2004; Buckingham and Rigby, 2014). Thus, MyoG is an important gene for muscle growth and repair. In the current study, carnosine administration showed a potential for muscle growth by enhancing MyoG levels of QMCs when cells were induced to undergo cell differentiation caused by reduced FBS concentration. However, reducing the FBS concentration to 2% in PMC did not induce a change in MyoG, thus failing to induce cell differentiation. Primary muscle cells are often more sensitive and require more precise control of culture conditions compared to established cell lines due to their limited lifespan and tendency to senesce over time. As a result, further investigation may be required to determine the optimal FBS concentration, satellite cell collection, and confluent ratio for primary muscle cell culture, which can influence their differentiation, proliferation, and gene expression.
To evaluate the anti-parasitic efficacy of carnosine, anti-coccidial effect was also assessed in the in vitro study. cNK-lysin, antimicrobial peptide which was used as a positive control exhibited a dose-dependent anticoccidial effect by reducing the viability of E. maxima sporozoites. cNK-lysin was discovered in Eimeria-infected chicken intestinal lymphocytes (Min et al., 2005; Hong et al., 2006a) based on its anti-parasitic activity against apicomplexan parasites such as Eimeria spp., via disrupting the parasitic membrane and modulating local inflammatory response (Lee et al., 2013). In contrast, carnosine administration did not demonstrate a direct anti-coccidial effect on E. maxima sporozoites.
The in vitro findings suggest that carnosine may exert potential benefits in chickens, as it demonstrates the ability to mitigate inflammation in CMCs following LPS stimulation and promote myogenic differentiation in QMCs.
In the in vivo study, dietary supplementation of young broiler chickens with carnosine did not affect BW and ADG in uninfected chickens. However, Eimeria challenge infection, irrespective of carnosine supplementation, induced a reduction in body weight of young chickens up to 10 to 14% at 6 dpi compared to noninfected chickens. Based on our experience, if the BW difference between the Eimeria-infected group and the noninfected group is typically around 10-15%, it is considered an appropriate level for evaluating the efficacy of feed additives against infection (Park et al., 2022a; b; Lee et al., 2022b; Wickramasuriya et al., 2023). To maintain this level, it is very important to accurately count the number of sporulated oocysts used in artificial infection and how much fresh oocyst is used after propagation. Too few or damaged oocysts make it difficult to show the effect of infection, and conversely, chickens infected with too many or very active oocysts cannot function with any feed additives because the degree of infection is too severe. At 8 dpi, the BW in chickens infected with E. maxima was further reduced to 25% in the PC group compared to the NC. However, supplementation of carnosine maintained the reduction of BW to around 15% in both HCS and LCS groups compared to that of the NC group. Like BW, ADG of chickens showed the same pattern. These results collectively suggest that carnosine could be used as a dietary supplement to mitigate the negative effects of E. maxima infection on growth of chickens. According to the literature, in the absence of infection, dietary carnosine supplementation within the range of 100 to 400 mg/kg feed showed a beneficial effect on the FCR of 21-day-old broiler chickens (Arbor Acres) (Cong et al., 2017). Additionally, dietary carnosine supplementation at 2.7 g/kg feed also exerted greater BW and feed intake of 28-day-old broiler chickens (Hubbard Flex) (Kopec et al., 2020). However, just like our study where carnosine showed no effect before the infection, the same was observed in other studies where dietary carnosine supplementation at 5 g/kg feed did not affect growth performance of broiler chicken (Arbor Acres) by 42-day-old (Hu et al., 2009). These inconsistencies in the observed outcomes of dietary carnosine effect in chickens may be attributed to factors such as the genetics and the age of chicken lines used as well as the concentration of carnosine in the diet. Limited research has been conducted to examine the impact of dietary carnosine supplementation under parasite infection, particularly regarding chicken coccidiosis. As such, this study represents the first documented evidence of improved growth performance in chickens fed carnosine supplementation following infection with E. maxima. The growth-promoting effect of dietary carnosine supplementation on E. maxima infection may be directly linked to the decrease in jejunal gut lesion and reduced parasite fecundity through an effective anti-parasite host response. Notably, despite in vitro experiments showing the absence of impact of carnosine on the viability of E. maxima sporozoites, dietary carnosine supplementation directly decreased jejunal lesion scores and oocyst shedding. However, there is a paucity of research on the anti-parasitic activity of carnosine, with only a few reports indicating reduced intrahepatic worm burden and egg count in hamsters with schistosomiasis (Soliman et al., 2001), rabbits with fascioliasis (Ali, 2012; Mohamed et al., 2013), and rats with trichinellosis (Soliman et al., 2007) following carnosine administration. Therefore, the underlying mechanism of the observed causal relationship between the growth-promoting effect of carnosine and the reduction of E. maxima oocyst may be related to the reduced intestinal inflammatory response and preservation of the intestinal integrity of chickens fed with dietary carnosine supplementation.
The infection with E. maxima led to an increase in jejunal permeability by decreasing the gene expression levels of claudin. In contrast, the HCS diet effectively restored the permeability by promoting the gene expression of claudin and occludin compared to those in the PC group. TJ proteins, such as claudins, occludin, and JAMs, form a complex network of strands that seal the paracellular space between neighboring cells (Groschwitz and Hogan, 2009; Jin and Blikslager, 2020; Furuse et al., 2023). This tight seal maintains the separation between the apical and basolateral domains, preventing the mixing of membrane components and regulating the passage of molecules between these compartments (Shi et al., 2018). Claudins are primarily responsible for the formation and selectivity of paracellular pores in tight junctions, while occludin plays a role in regulating barrier function, cell signaling, and the stability of tight junctions (Overgaard et al., 2011; Tsukita et al., 2019; Saito et al., 2021).
E. maxima infection in chickens affects the epithelial cells of the jejunum in various ways, including cell destruction Ghareeb et al., 2022), inflammation (Park et al., 2021), altered tight junctions (Teng et al., 2020), reduced expression of nutrient transporters (Teng et al., 2021a), oxidative stress (Khatlab et al., 2019), and malabsorption (Ghareeb et al., 2022). These factors contribute to the impaired function of nutrient transporters and a decrease in the overall nutrient uptake in the infected birds. In this study, we measured various nutritional transporters in the jejunum. However, unlike inflammatory cytokine responses and tight junction proteins which impaired following coccidiosis infection, there were no significant changes in most of the nutritional transporters. It is likely that the mild coccidiosis infection model that we used in this study did not allow to observe significant changes in most of the nutritional transporter associated to coccidiosis. This result is supported by several previous studies that have shown similar findings (Teng et al., 2020,2021a,b). Therefore, it is evident that the severity of coccidiosis infection model that are used in animal studies is of great importance to see the effect of dietary factors. Supplementation of carnosine at 10 mg/kg resulted in a significant increase in the gene expression levels of B0AT and B0+AT, with more than a 5-fold upregulation compared to the uninfected group. Additionally, SGLT and LAT1 expression levels were also elevated more than 3-fold in the carnosine-supplemented group at 10 mg/kg compared to the infected group without carnosine supplementation. B0AT, also known as SLC6A19, is a sodium-dependent neutral amino acid transporter found in the jejunum of chickens. It transports various neutral (noncharged) amino acids such as leucine, isoleucine, valine, methionine, phenylalanine, and tryptophan (Bröer, 2009; Stevens, 2010; Wong, 2022). B0AT transporters have a broad substrate specificity for neutral amino acids and play a vital role in the absorption of these amino acids in the jejunum (Stevens, 2010). B0+AT, also known as SLC6A14, is another sodium-dependent amino acid transporter expressed in the jejunum of chickens (Mastrototaro et al., 2016; Nałęcz, 2020). It has a broader substrate specificity compared to B0AT, as it can transport not only neutral amino acids but also positively charged (cationic) amino acids such as arginine, lysine, and histidine (Stevens, 2010; Mastrototaro et al., 2016). B0+AT is essential for the absorption of both neutral and cationic amino acids in the jejunum (Li et al., 2008). This difference in substrate specificity affects their roles in amino acid absorption in the jejunum and their overall contribution to maintaining the balance of amino acids in chickens. Both transporters are essential for efficient nutrient absorption, and their function can be influenced by various factors, such as diet, age, and health status of the chickens. In the jejunum of chickens, SGLT transporters are predominantly expressed in the brush border membrane of the epithelial cells lining the intestine (Gopi et al., 2023). Their primary function of SGLT is to facilitate glucose absorption from the digested feed, ensuring that the chicken's body receives an adequate supply of glucose for energy production and other metabolic processes (Awad et al., 2014). The primary role of LAT1 is to mediate the transport of large neutral amino acids, including leucine, isoleucine, valine, phenylalanine, tryptophan, and methionine (Wang et al., 2022; Abdel-Raheem et al., 2023). In our study, these results were not as clear as we expected, but the dietary supplementation of carnosine at 10 mg/kg showed a tendency to induce beneficial effects on the expression of amino acid and glucose transporters in the jejunum of chickens infected with E. maxima, compared to chickens infected with E. maxima without carnosine supplementation. This suggests that nutrient absorption and overall intestinal health could be improved upon dietary carnosine supplementation.
Based on the in vivo experimental results, although dietary carnosine supplementation did not yield significant effects in the uninfected groups, it notably enhanced the body weight and the average daily gain in Eimeria-infected group compared to the Eimeria-infected group without carnosine supplementation. This beneficial effect could be attributed to the lower gut lesion scores and reduced parasite fecundity observed in the carnosine-supplemented group. Interestingly, while carnosine administration did not directly reduce the number of sporozoites of E. maxima in vitro, dietary carnosine supplementation did decrease fecal oocyst shedding of E. maxima in vivo. This effect could be due to the suppression of local inflammatory cytokine secretion by carnosine at 10 mg/kg. Furthermore, the carnosine-supplemented group exhibited a recovery in tight junction protein levels following coccidiosis, accompanied by an increased activity in the nutritional transporter levels. This suggests that the intestinal homeostasis was attempting to maintain equilibrium, leading to improved gut health in the carnosine-supplemented group compared to coccidiosis-infected group without supplementation. Ultimately, these findings imply that the carnosine supplementation enhanced gut health and improved broiler growth, and mitigated coccidiosis infection.
In conclusion, integrating the findings from both in vitro and in vivo studies, carnosine dietary administration alleviated LPS-induced inflammatory responses in chicken macrophage cells and promoted muscle cell differentiation in vitro. In the in vivo study, dietary carnosine supplementation effectively strengthened gut integrity, mitigated parasite-induced intestinal damage, and reduced parasite fecundity, thereby mitigating coccidiosis-induced growth impairment in broiler chickens infected with E. maxima. Therefore, the hypothesis that carnosine supplementation can alter the inflammatory response, permeability, nutrient transport, and growth performance of broilers infected with E. maxima has been proven through results of this study. Furthermore, this study demonstrates the beneficial effects of dietary supplementation of intestinal metabolites such as carnosine which can be identified through global metabolomics, and potential use of beneficial gut metabolites as novel alternatives to growth promoting antibiotics to mitigate negative effects of devastating parasitic infection such as coccidiosis in young broiler chickens.
DISCLOSURES
Author AHS and TGR were employed by the Arm & Hammer Animal and Food Production. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ACKNOWLEDGMENTS
Funding was mainly supported by ARS CRIS 8042-32000-115-00D, and partly by USDA/NIFA SAS award 2020-69012-31823 and ARS Material Transfer Research Agreement, #58-8042-0-046.
Data Availability Statement: The datasets generated in this study are available upon request to the corresponding author.
Ethics Statement: The animal use and experimental protocols were reviewed and approved by the Beltsville Agricultural Research Center Small Animal Care Committee (Animal Protocol No. 19–018).
Author Contributions: IP and HSL designed the research. HSL supervised the research and edited the manuscript. IP, and HN conducted the in vitro research. IP, YL, and SW conducted in vivo research. IP analyzed data and drafted the manuscript. AHS edited the manuscript. IP, HN, YL, SW, AHS, TGR, and HSL had responsibility for the content.
REFERENCES
- Abd El-Hack M.E., El-Saadony M.T., Salem H.M., El-Tahan A.M., Soliman M.M., Youssef G.B.A., Taha A.E., Soliman S.M., Ahmed A.E., El-kott A.F., Al Syaad K.M., Swelum A.A. Alternatives to antibiotics for organic poultry production: types, modes of action and impacts on bird's health and production. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Raheem S.M., Mohammed E.S.Y., Mahmoud R.E., El Gamal M.F., Nada H.S., El-Ghareeb W.R., Marzok M., Meligy A.M.A., Abdulmohsen M., Ismail H., Ibrahim D., Kishawy A.T.Y. Double-fermented soybean meal totally replaces soybean meal in broiler rations with favorable impact on performance, digestibility, amino acids transporters and meat nutritional value. Animals (Basel) 2023;13:1030. doi: 10.3390/ani13061030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajibola O.O., Thomas R., Bakare B.F. Selected fermented indigenous vegetables and fruits from Malaysia as potential sources of natural probiotics for improving gut health. Food Sci. Hum. Wellness. 2023;12:1493–1509. [Google Scholar]
- Ali S.A. Evaluation of the immunological effect of beta alanyl-l-histidine against Schistosoma mansoni antigens in rabbits. J. Infect. Dev. Ctries. 2012;6:166–175. doi: 10.3855/jidc.1549. [DOI] [PubMed] [Google Scholar]
- Arenas-Gómez C.M., Garcia-Gutierrez E., Escobar J.S., Cotter P.D. Human gut homeostasis and regeneration: the role of the gut microbiota and its metabolites. Crit. Rev. Microbiol. 2023;49:764–785. doi: 10.1080/1040841X.2022.2142088. [DOI] [PubMed] [Google Scholar]
- Awad W.A., Aschenbach J.R., Ghareeb K., Khayal B., Hess C., Hess M. Campylobacter jejuni influences the expression of nutrient transporter genes in the intestine of chickens. Vet. Microbiol. 2014;172:195–201. doi: 10.1016/j.vetmic.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Boldyrev A.A., Aldini G., Derave W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013;93:1803–1845. doi: 10.1152/physrev.00039.2012. [DOI] [PubMed] [Google Scholar]
- Bröer S. The role of the neutral amino acid transporter B0AT1 (SLC6A19) in hartnup disorder and protein nutrition. IUBMB Life. 2009;61:591–599. doi: 10.1002/iub.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugaletta G., De Cesare A., Laghi L., Manfreda G., Zampiga M., Oliveri C., Pérez-Calvo E., Litta G., Lolli S., Sirri F. A multi-omics approach to elucidate the mechanisms of action of a dietary muramidase administered to broiler chickens. Sci. Rep. 2022;12:5559. doi: 10.1038/s41598-022-09546-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M., Rigby P.W.J. Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev. Cell. 2014;28:225–238. doi: 10.1016/j.devcel.2013.12.020. [DOI] [PubMed] [Google Scholar]
- Cong J., Zhang L., Li J., Wang S., Gao F., Zhou G. Effects of dietary supplementation with carnosine on growth performance, meat quality, antioxidant capacity and muscle fiber characteristics in broiler chickens. J. Sci. Food Agric. 2017;97:3733–3741. doi: 10.1002/jsfa.8236. [DOI] [PubMed] [Google Scholar]
- Cornelison, D. D. W., and B. J. Wold. 1997. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. 191:270-83. [DOI] [PubMed]
- Davison G., Marchbank T., March D.S., Thatcher R., Playford R.J. Zinc carnosine works with bovine colostrum in truncating heavy exercise-induced increase in gut permeability in healthy volunteers. Am. J. Clin. Nutr. 2016;104:526–536. doi: 10.3945/ajcn.116.134403. [DOI] [PubMed] [Google Scholar]
- Fleisher-Berkovich S., Abramovitch-Dahan C., Ben-Shabat S., Apte R., Beit-Yannai E. Inhibitory effect of carnosine and N-acetyl carnosine on LPS-induced microglial oxidative stress and inflammation. Peptides. 2009;30:1306–1312. doi: 10.1016/j.peptides.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Fresta C.G., Fidilio A., Lazzarino G., Musso N., Grasso M., Merlo S., Amorini A.M., Bucolo C., Tavazzi B., Lazzarino G., Lunte S.M., Caraci F., Caruso G. Modulation of pro-oxidant and pro-inflammatory activities of m1 macrophages by the natural dipeptide carnosine. Int. J. Mol. Sci. 2020;21:776. doi: 10.3390/ijms21030776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M., Nakatsu D., Hempstock W., Sugioka S., Ishizuka N., Furuse K., Sugawara T., Fukazawa Y., Hayashi H. Reconstitution of functional tight junctions with individual claudin subtypes in epithelial cells. Cell Struct. Funct. 2023;48:1–17. doi: 10.1247/csf.22068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadde U., Kim W.H., Oh S.T., Lillehoj H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim. Health Res. Rev. 2017;18:26–45. doi: 10.1017/S1466252316000207. [DOI] [PubMed] [Google Scholar]
- Ghareeb A.F.A., Schneiders G.H., Richter J.N., Foutz J.C., Milfort M.C., Fuller A.L., Yuan J., Rekaya R., Aggrey S.E. Heat stress modulates the disruptive effects of Eimeria maxima infection on the ileum nutrient digestibility, molecular transporters, and tissue morphology in meat-type chickens. PLoS One. 2022;17 doi: 10.1371/journal.pone.0269131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopi M., Rokade J.J., Kolluri G., S T.S., Prabakar G., Madhupriya V., Tyagi J.S. Supplementary nucleosides improve the performance through enhanced enzyme activity, intestinal development and transporter genes in broiler chickens. Nucleosides Nucleotides Nucleic Acids. 2023;42:547–562. doi: 10.1080/15257770.2023.2169454. [DOI] [PubMed] [Google Scholar]
- Granstad S., Kristoffersen A.B., Benestad S.L., Sjurseth S.K., David B., Sørensen L., Fjermedal A., Edvardsen D.H., Sanson G., Løvland A., Kaldhusdal M. Effect of feed additives as alternatives to in-feed antimicrobials on production performance and intestinal Clostridium perfringens counts in broiler chickens. Animals (Basel) 2020;10:240. doi: 10.3390/ani10020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groschwitz K.R., Hogan S.P. Intestinal barrier function: molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009;124:3–20. doi: 10.1016/j.jaci.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevy O., Piestun Y., Allouh M.Z., Rosser B.W.C., Rinkevich Y., Reshef R., Rozenboim I., Wleklinski-Lee M., Yablonka-Reuveni Z. Pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. Dev. Dyn. 2004;231:489–502. doi: 10.1002/dvdy.20151. [DOI] [PubMed] [Google Scholar]
- Hassan A., Ahn J., Suh Y., Choi Y.M., Chen P., Lee K. Selenium promotes adipogenic determination and differentiation of chicken embryonic fibroblasts with regulation of genes involved in fatty acid uptake, triacylglycerol synthesis and lipolysis. J. Nutr. Biochem. 2014;25:858–867. doi: 10.1016/j.jnutbio.2014.03.018. [DOI] [PubMed] [Google Scholar]
- Haug A., Thebo P., Mattsson J.G. A simplified protocol for molecular identification of Eimeria species in field samples. Vet. Parasitol. 2007;146:35–45. doi: 10.1016/j.vetpar.2006.12.015. [DOI] [PubMed] [Google Scholar]
- He Y., Maltecca C., Tiezzi F. Potential use of gut microbiota composition as a biomarker of heat stress in monogastric species: a review. Animals (Basel) 2021;11:1833. doi: 10.3390/ani11061833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y.H., Lillehoj H.S., Dalloul R.A., Min W., Miska K.B., Tuo W., Lee S.H., Han J.Y., Lillehoj E.P. Molecular cloning and characterization of chicken NK-lysin. Vet. Immunol. Immunopathol. 2006;110:339–347. doi: 10.1016/j.vetimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Hu X., Hongtrakul K., Ji C., Ma Q., Guan S., Song C., Zhang Y., Zhao L. Effect of carnosine on growth performance, carcass characteristics, meat quality and oxidative stability in broiler chickens. J. Poult. Sci. 2009;46:296–302. [Google Scholar]
- Jin Y., Blikslager A.T. The regulation of intestinal mucosal barrier by myosin light chain Kinase/Rho kinases. Int. J. Mol. Sci. 2020;21:3550. doi: 10.3390/ijms21103550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukić I., Kolobarić N., Stupin A., Matić A., Kozina N., Mihaljević Z., Mihalj M., Šušnjara P., Stupin M., Ćurić Ž.B., Selthofer-Relatić K., Kibel A., Lukinac A., Kolar L., Kralik G., Kralik Z., Széchenyi A., Jozanović M., Galović O., Medvidović-Kosanović M., Drenjančević I. Carnosine, small but mighty—prospect of use as functional ingredient for functional food formulation. Antioxidants (Basel) 2021;10:1037. doi: 10.3390/antiox10071037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatlab A.D.S., Del Vesco A.P., De Oliveira Neto A.R., Fernandes R.P.M., Gasparino E. Dietary supplementation with free methionine or methionine dipeptide mitigates intestinal oxidative stress induced by Eimeria spp. challenge in broiler chickens. J. Anim. Sci. Biotechnol. 2019;10:58. doi: 10.1186/s40104-019-0353-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W.H., Jeong J., Park A.R., Yim D., Kim S., Chang H.H., Yang S.H., Kim D.H., Lillehoj H.S., Min W. Downregulation of chicken interleukin-17 receptor A during Eimeria infection. Infect. Immun. 2014;82:3845–3854. doi: 10.1128/IAI.02141-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W.H., Lillehoj H.S., Min W. Evaluation of the immunomodulatory activity of the chicken NK-lysin-derived peptide cNK-2. Sci. Rep. 2017;7:45099. doi: 10.1038/srep45099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec W., Jamroz D., Wiliczkiewicz A., Biazik E., Pudlo A., Korzeniowska M., Hikawczuk T., Skiba T. Antioxidative characteristics of chicken breast meat and blood after diet supplementation with carnosine, l-histidine, and β-alanine. Antioxidants (Basel) 2020;9:1093. doi: 10.3390/antiox9111093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota M., Kobayashi N., Sugizaki T., Shimoda M., Kawahara M., Tanaka K.I. Carnosine suppresses neuronal cell death and inflammation induced by 6-hydroxydopamine in an in vitro model of Parkinson's disease. PLoS One. 2020;15 doi: 10.1371/journal.pone.0240448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Lillehoj H.S., Tuo W., Murphy C.A., Hong Y.H., Lillehoj E.P. Parasiticidal activity of a novel synthetic peptide from the core α-helical region of NK-lysin. Vet. Parasitol. 2013;197:113–121. doi: 10.1016/j.vetpar.2013.04.020. [DOI] [PubMed] [Google Scholar]
- Lee W.J., Hase K. Gut microbiota-generated metabolites in animal health and disease. Nat. Chem. Biol. 2014;10:416–424. doi: 10.1038/nchembio.1535. [DOI] [PubMed] [Google Scholar]
- Lee K.W., Lillehoj H.S., Kim W., Park I., Li C., Lu M., Hofacre C.L. Research Note: First report on the detection of necrotic enteritis (NE) B-like toxin in biological samples from NE-afflicted chickens using capture enzyme-linked immunosorbent assay. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Lu M., Lillehoj H.S. Coccidiosis: recent progress in host immunity and alternatives to antibiotic strategies. Vaccines (Basel) 2022;10:215. doi: 10.3390/vaccines10020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Park I., Wickramasuriya S.S., Ben Arous J., Koziol M.E., Lillehoj H.S. Co-administration of chicken IL-7 or NK-lysin peptide 2 enhances the efficacy of Eimeria elongation factor-1α vaccination against Eimeria maxima infection in broiler chickens. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levatte M., Keshteli A.H., Zarei P., Wishart D.S. Applications of metabolomics to precision nutrition. Lifestyle Genom. 2022;15:1–9. doi: 10.1159/000518489. [DOI] [PubMed] [Google Scholar]
- Li H., Gilbert E.R., Zhang Y., Crasta O., Emmerson D., Webb K.E., Wong E.A. Expression profiling of the solute carrier gene family in chicken intestine from the late embryonic to early post-hatch stages. Anim. Genet. 2008;39:407–424. doi: 10.1111/j.1365-2052.2008.01744.x. [DOI] [PubMed] [Google Scholar]
- Lillehoj H.S., Lee S.H., Park S.S., Jeong M., Lim Y., Mathis G.F., Lumpkins B., Chi F., Ching C., Cravens R.L. Calcium montmorillonite-based dietary supplement attenuates necrotic enteritis induced by Eimeria maxima and Clostridium perfringens in broilers. J. Poult. Sci. 2016;53:329–340.. doi: 10.2141/jpsa.0150182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone K.M., Ramos-Lopez O., Pérusse L., Kato H., Ordovas J.M., Martínez J.A. Precision nutrition: a review of current approaches and future endeavors. Trends Food Sci. Technol. 2022;128:253–264. [Google Scholar]
- Mahfuz S., Shang Q., Piao X. Phenolic compounds as natural feed additives in poultry and swine diets: a review. J. Anim. Sci. Biotechnol. 2021;12:48. doi: 10.1186/s40104-021-00565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood A., FitzGerald A.J., Marchbank T., Ntatsaki E., Murray D., Ghosh S., Playford R.J. Zinc carnosine, a health food supplement that stabilises small bowel integrity and stimulates gut repair processes. Gut. 2007;56:168–175. doi: 10.1136/gut.2006.099929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrototaro L., Sponder G., Saremi B., Aschenbach J.R. Gastrointestinal methionine shuttle: priority handling of precious goods. IUBMB Life. 2016;68:924–934. doi: 10.1002/iub.1571. [DOI] [PubMed] [Google Scholar]
- Min W., Lillehoj H.S., Ashwell C.M., van Tassel C.P., Dalloul R.A., Matukumalli L.K., Han J.Y., Lillehoj E.P. Expressed sequence tag analysis of Eimeria-stimulated intestinal intraepithelial lymphocytes in chickens. Mol. Biotechnol. 2005;30:143–150. doi: 10.1385/MB:30:2:143. [DOI] [PubMed] [Google Scholar]
- Mohamed N.Z., El Kader M.A.A., Ali S.A. Efficacy of L-carnosine against Schistosoma mansoni antigens in liver, heart, kidney and brain of rabbits. J. Appl. Pharm. Sci. 2013;3:39–45. [Google Scholar]
- Nałęcz K.A. Amino acid transporter SLC6A14 (ATB0,+) – a target in combined anti-cancer therapy. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.594464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard C.E., Daugherty B.L., Mitchell L.A., Koval M. Claudins: control of barrier function and regulation in response to oxidant stress. Antioxid. Redox Signal. 2011;15:1179–1193. doi: 10.1089/ars.2011.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park I., Goo D., Nam H., Wickramasuriya S.S., Lee K., Zimmerman N.P., Smith A.H., Rehberger T.G., Lillehoj H.S. Effects of dietary maltol on innate immunity, gut health, and growth performance of broiler chickens challenged with Eimeria maxima. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.667425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park I., Lee Y., Goo D., Zimmerman N.P., Smith A.H., Rehberger T., Lillehoj H.S. The effects of dietary Bacillus subtilis supplementation, as an alternative to antibiotics, on growth performance, intestinal immunity, and epithelial barrier integrity in broiler chickens infected with Eimeria maxima. Poult. Sci. 2020;99:725–733. doi: 10.1016/j.psj.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park I., Nam H., Goo D., Wickramasuriya S.S., Zimmerman N., Smith A.H., Rehberger T.G., Lillehoj H.S. Gut microbiota-derived indole-3-carboxylate influences mucosal integrity and immunity through the activation of the aryl hydrocarbon receptors and nutrient transporters in broiler chickens challenged with Eimeria maxima. Front. Immunol. 2022;13:1–16. doi: 10.3389/fimmu.2022.867754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park I., Oh S., Goo D., Celi P., Lillehoj H.S. Effect of dietary sophorolipids on growth performance and gastrointestinal functionality of broiler chickens infected with Eimeria maxima. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park I., Zimmerman N.P., Smith A.H., Rehberger T.G., Lillehoj E.P., Lillehoj H.S. Dietary supplementation with Bacillus subtilis direct-fed microbials alters chicken intestinal metabolite levels. Front. Vet. Sci. 2020;7:123. doi: 10.3389/fvets.2020.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A.C., Higashi T., Fukazawa Y., Otani T., Tauchi M., Higashi A.Y., Furuse M., Chiba H. Occludin and tricellulin facilitate formation of anastomosing tight-junction strand network to improve barrier function. Mol. Biol. Cell. 2021;32:722–738. doi: 10.1091/mbc.E20-07-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura-Wang M., Grabner N., Koestelbauer A., Klose V., Ghanbari M. Genome-resolved metagenomics of the chicken gut microbiome. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.726923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehata A.A., Yalçın S., Latorre J.D., Basiouni S., Attia Y.A., El-Wahab A.A., Visscher C., El-Seedi H.R., Huber C., Hafez H.M., Eisenreich W., Tellez-Isaias G. Probiotics, prebiotics, and phytogenic substances for optimizing gut health in poultry. Microorganisms. 2022;10:395. doi: 10.3390/microorganisms10020395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Barakat M., Chen D., Chen L. Bicellular tight junctions and wound healing. Int. J. Mol. Sci. 2018;19:3862. doi: 10.3390/ijms19123862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S., Choi Y.M., Suh Y., Lee K. Delta-like 1 homolog (DLK1) inhibits proliferation and myotube formation of avian QM7 myoblasts. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2015;179:37–43. doi: 10.1016/j.cbpb.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Soliman K.M., Abdul-Hamid M., Othman A.I. Effect of carnosine on gentamicin-induced nephrotoxicity. Med. Sci. Monit. 2007;13:BR73–BR83. [PubMed] [Google Scholar]
- Soliman K., El-Ansary A., Mohamed A.M. Effect of carnosine administration on metabolic parameters in bilharzia-infected hamsters. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001;129:157–164. doi: 10.1016/s1096-4959(01)00332-3. [DOI] [PubMed] [Google Scholar]
- Stevens B.R. In: Pages 353–378 in Epithelial Transport Physiology. Gerencser G.A., editor. Humana Press Inc; Totowa, NJ: 2010. Amino acid transport by epithelial membranes. [Google Scholar]
- Sun X., Wang Z., Shao C., Yu J., Liu H., Chen H., Li L., Wang X., Ren Y., Huang X., Zhang R., Li G. Analysis of chicken macrophage functions and gene expressions following infectious bronchitis virus M41 infection. Vet. Res. 2021;52:14. doi: 10.1186/s13567-021-00896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebani A., Bekri S. Paving the way to precision nutrition through metabolomics. Front Nutr. 2019;6:41. doi: 10.3389/fnut.2019.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng P.Y., Choi J., Tompkins Y., Lillehoj H., Kim W. Impacts of increasing challenge with Eimeria maxima on the growth performance and gene expression of biomarkers associated with intestinal integrity and nutrient transporters. Vet. Res. 2021;52:81. doi: 10.1186/s13567-021-00949-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng P.Y., Yadav S., de S. Castro F.L., Tompkins Y.H., Fuller A.L., Kim W.K. Graded Eimeria challenge linearly regulated growth performance, dynamic change of gastrointestinal permeability, apparent ileal digestibility, intestinal morphology, and tight junctions of broiler chickens. Poult. Sci. 2020;99:4203–4216. doi: 10.1016/j.psj.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng P.Y., Yadav S., Shi H., Kim W.K. Evaluating endogenous loss and standard ileal digestibility of amino acids in response to the graded severity levels of E. maxima infection. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S., Tanaka H., Tamura A. The claudins: from tight junctions to biological systems. Trends Biochem. Sci. 2019;44:141–152. doi: 10.1016/j.tibs.2018.09.008. [DOI] [PubMed] [Google Scholar]
- Urgessa O.E., Woldesemayat A.A. OMICs approaches and technologies for understanding low-high feed efficiency traits in chicken: implication to breeding. Anim. Biotechnol. 2023;16:1–20. doi: 10.1080/10495398.2023.2187404. [DOI] [PubMed] [Google Scholar]
- Wang G., Li X., Zhou Y., Feng J., Zhang M. Effects of dietary chromium picolinate on gut microbiota, gastrointestinal peptides, glucose homeostasis, and performance of heat-stressed broilers. Animals (Basel) 2022;12:844. doi: 10.3390/ani12070844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickramasuriya S.S., Park I., Lee K., Lee Y., Kim W.H., Nam H., Lillehoj H.S. Role of physiology, immunity, microbiota, and infectious diseases in the gut health of poultry. Vaccines (Basel) 2022;10:172. doi: 10.3390/vaccines10020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickramasuriya S.S., Park I., Lee Y., Richer L.M., Przybyszewski C., Gay C.G., van Oosterwijk J.G., Lillehoj H.S. Orally delivered bacillus subtilis expressing chicken NK-2 peptide stabilizes gut microbiota and enhances intestinal health and local immunity in coccidiosis-infected broiler chickens. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E.A. In: Pages 529–548 in Stukie’s Avian Physiology. Scanes C.G., Dridi S., editors. Academic Press; Cambridge, MA, USA: 2022. Functional properties of avian intestinal cells. [Google Scholar]
- Wu G. Important roles of dietary taurine, creatine, carnosine, anserine and 4-hydroxyproline in human nutrition and health. Amino Acids. 2020;52:329–360. doi: 10.1007/s00726-020-02823-6. [DOI] [PMC free article] [PubMed] [Google Scholar]