Figure 3.
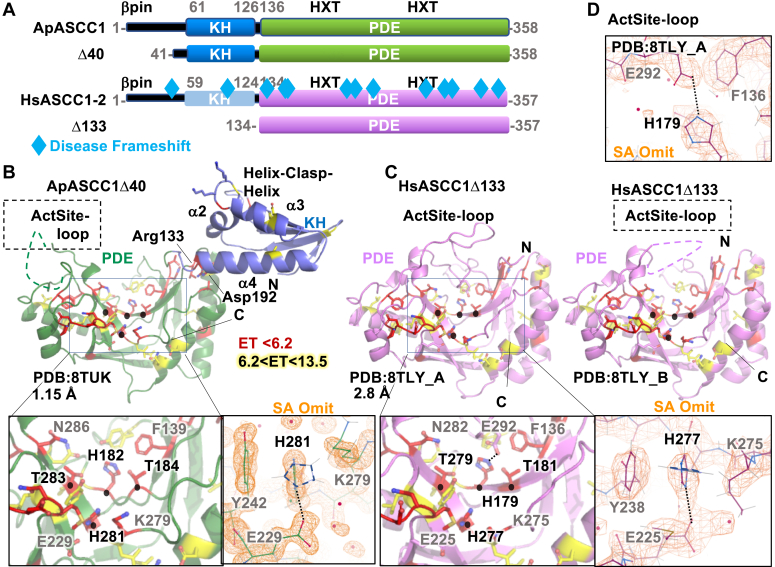
ASCC1 has tandem KH and PDE domains with uncommon Histidine rotamer and an active site loop.A, domain schematic of Alvinella (Ap) and Human (Hs, Isoform 2) ASCC1 and constructs used in this study. ASCC1 germline mutations that cause frameshifts in ASCC1 (Table S1) are mapped onto the human schematic. B, the crystal structure of ApASCC1Δ40 shows the relative position of the KH and PDE domains and their functional motifs. The two HXT motifs (black spheres) cluster in adjacent anti-parallel beta strands at the bottom of the V-shaped groove in the PDE domain on the same side as the phosphate-interacting GKKG motif in the KH domain. Conserved residues ranked by ET analysis localize to the PDE 2HXT groove, the hinge, and the KH domain. Left zoom inset shows conserved residues in the PDE including the HXT motifs. Right panel with simulated Annealing (SA) omit maps (orange, contoured at 1.5s) shows an uncommon rotamer position of the Histidine in the second HXT motif due to steric sandwiching between a Tyr and Lys and hydrogen bonded to a glutamate. The overlaid His side chain outline (dashed) shows the closest preferred rotamer. C, crystal structures of HsASCC1Δ133 reveal functional motifs in the PDE domain, depicted as in B. There were two molecules in the asymmetric unit (chain A and B). The comparison revealed an active site loop coordinated to the first HXT motif and that is only present in the A chain. D, ActSite-Loop coordination to His179 in the first HXT motif, overlaid with SA omit map (orange, contoured at 1.5s).