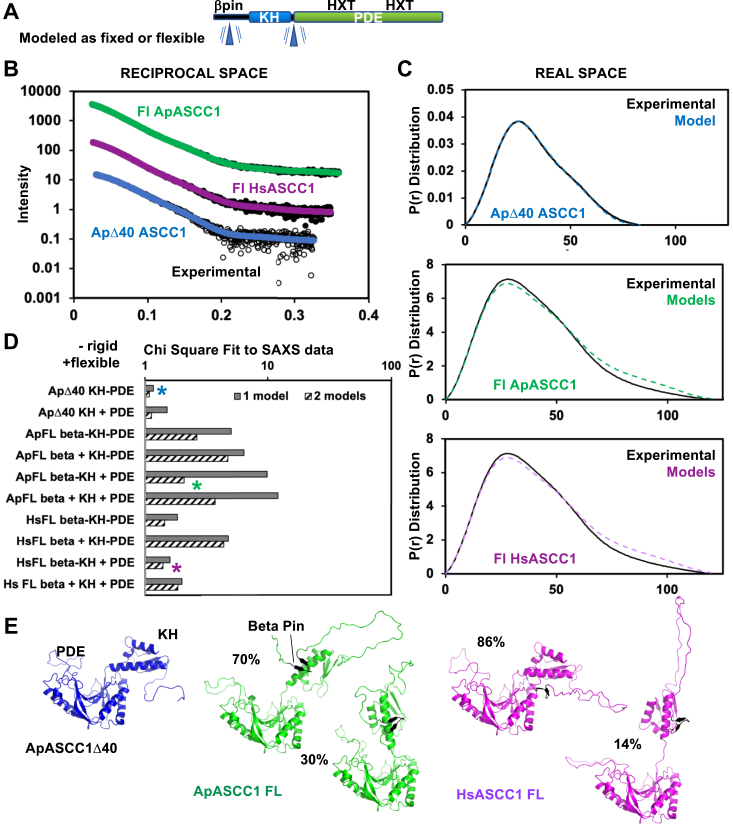
Figure 7.
SAXS analysis indicates stabilized KH-PDE domains with the potential for flexibility and supports the betapin model.A, ASCC1 domain schematic indicating with a blue triangle which regions were allowed to be flexible in the SAXS modeling. B, experimental reciprocal space SAXS data (black) for ApASCC1Δ40, ApASCC1 full-length (FL), and HsASCC1 FL SAXS curves predicted from best-fitting models (colored, see C) are overlaid. Initial models were built based on crystal structures and AlphaFold2 models and fit to the experimental SAXS data. C, experimental SAXS data and models, as described in (B), in real space. D, lowest chi square metrics of SAXS-fit atomic models to experimental SAXS data. During modeling, beta pin, KH, and PDE domains were internally kept rigid, but the domains relative to each other were either kept rigid (−) or allowed to move (+), as indicated by blue triangle in (A). Models (∗) with the lowest chi square were selected for further analysis. E, best SAXS-fit atomic models were consistent with ASCC1 dominant KH-PDE conformation being similar to the crystal orientation, potential for limited flexibility between KH and PDE domains, and a beta pin predicted by AlphaFold2 to reside next to the KH domain.