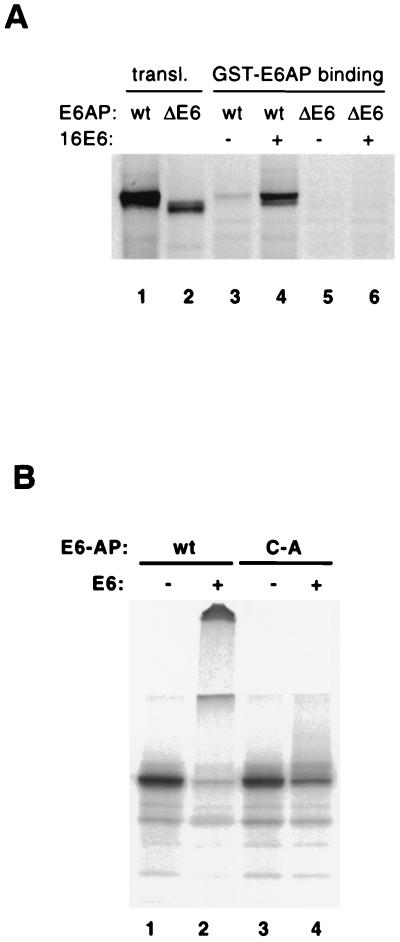
FIG. 7.
HPV16 E6 induces degradation of a mixture of wild-type and mutant E6AP and mediates E6AP dimerization in vitro. (A) E6AP dimerization in the presence of E6. In vitro-translated, 35S-labeled wild-type (lanes 3 and 4) and ΔE6 (lanes 5 and 6) forms of E6AP were incubated with GST-E6AP protein in the presence or absence of E6 and then immobilized on glutathione-Sepharose beads. The beads were washed and analyzed by SDS-PAGE, followed by autoradiography. (B) Degradation of wild-type and mutant E6AP in vitro. E6AP wild type (wt) and the active-site Cys-to-Ala mutant (C-A) were translated in rabbit reticulocyte lysate in the presence of [35S]methionine. The translation reactions were incubated for 15 min at room temperature with E1 protein, E2 protein (A. thaliana Ubc8), ubiquitin, ATP, and baculovirus-expressed HPV16 E6 protein (+) or an equivalent control fraction (−). Reaction products were analyzed by SDS-PAGE and autoradiography.