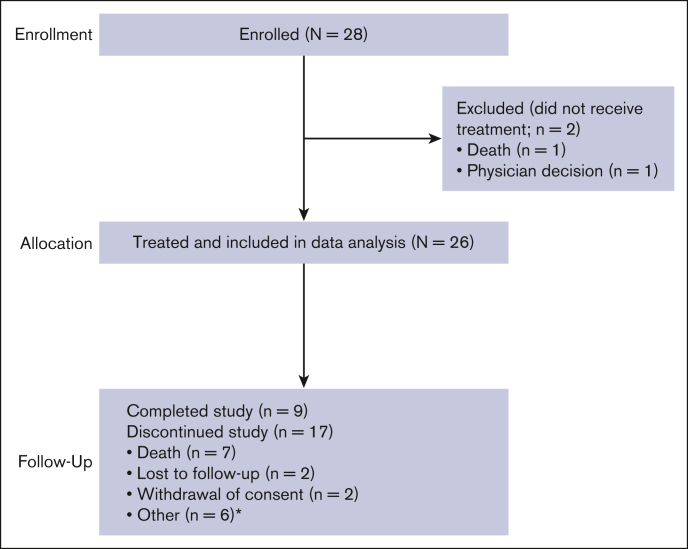
Figure 1.
Study disposition. ∗Removed from study by sponsor because of concurrent cytotoxic T-lymphocyte treatment with different agent for cytomegalovirus disease (n = 1); primary disease relapse (n = 1); patient noncompliance with follow-up appointments (n = 1); patient exiting study 5 months after treatment discontinuation because of start of subsequent therapy (n = 1); patient enrolling on different protocol (n = 1); and physician decision (n = 1).