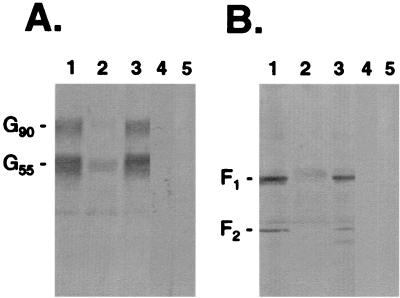
FIG. 1.
Heparin-agarose affinity chromatography of RSV (strain A2)-infected Vero cell lysates. HAAC was carried out on infected cell lysates (250 μg of protein per ml of heparin-agarose) followed by immunoprecipitation. Samples were analyzed by SDS-PAGE (4 to 20% gels) followed by Western blot with a polyclonal rabbit anti-G (A) or anti-F (B) antiserum. The infected cell lysate was analyzed for the presence of RSV-specific protein (lane 1) prior to being run over heparin-agarose columns. Columns were washed twice with 10 column volumes of MES buffer, and the final wash was examined (lane 2). Bound proteins were eluted with MES buffer containing heparin (2 mg/ml) (lane 3). As a control to demonstrate that the viral proteins were specifically binding to heparin, identical samples were run over columns containing uncoupled CL-4B agarose, and the heparin-eluted material was examined for the presence of RSV-G and -F proteins (lane 4). In addition, uninfected cell lysate was run over heparin-agarose to control for polyclonal antibody reactivity (lane 5). G90 represents the mature, fully glycosylated form of RSV-G. G55 is a partially glycosylated precursor of G90.