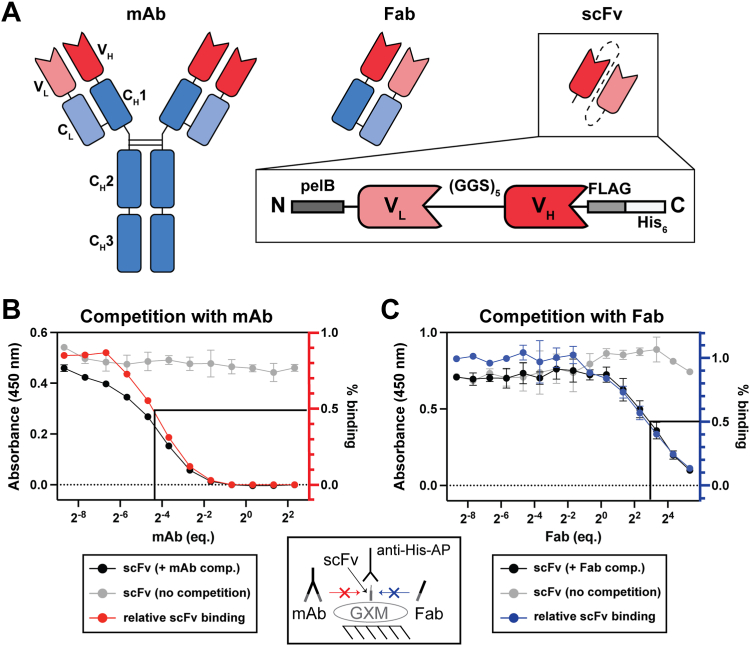
Figure 1.
Single-chain Fv antibody fragment 18B7 competes for binding to GXM antigen with corresponding mAb.A, schematic depicting the antibody fragments used in this study. Variable domains are colored red and constant domains in blue. Light chains are a lighter hue than heavy chains. Inset, map of scFv constructs used in this study: domains are in the VL-VH arrangement, connected by a 15-residue glycine-serine linker with FLAG and His6 peptide tags are appended to the C-terminus. B and C, competition for binding of mAb or Fab 18B7 and scFv 18B7 to C. neoformans strain H99 exopolysaccharide was assessed by ELISA competition experiments. In both experiments, a constant concentration of scFv competed for binding with an increasing concentration gradient of mAb or Fab 18B7 and bound scFv was measured at each point to determine binding inhibition. Data points for scFv binding with and without competition are colored black, gray. Data represent the mean ± SD of two replicate measurements. The molar equivalents of mAb or Fab that achieved 50% binding inhibition are indicated on the plots. B, the binding of antigen by scFv 18B7 at a constant concentration with respect to a gradient of competing mAb 18B7 was determined. The percentage of uninhibited scFv binding to antigen at each mAb concentration is plotted in red. C, the binding of antigen by scFv 18B7 at a constant concentration with respect to a gradient of competing Fab 18B7 was determined. The percentage of uninhibited scFv binding to antigen at each Fab concentration is plotted in blue.