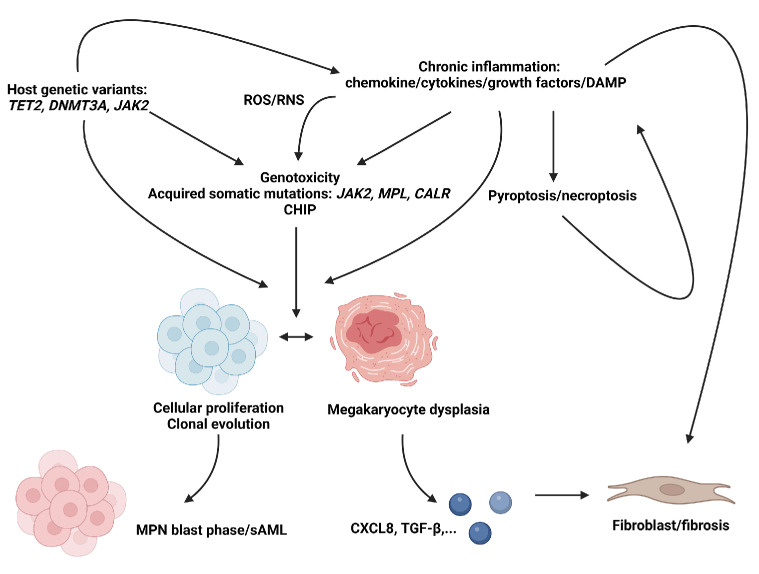
Figure 2.
Overview of primary myelofibrosis pathophysiology. The role of inherited genetic variants is incompletely understood in primary myelofibrosis (PMF) and other BCR::ABL1 negative myeloproliferative neoplasms (MPN) but may contribute to an increased susceptibility of acquiring somatic mutations or chronic inflammatory states. Acquired somatic mutations may occur in key driver genes encoding Janus Kinase 2 (JAK2), the thrombopoietin receptor (MPL) or calreticulin (CALR) or may contribute to the development of clonal hematopoiesis of intermediate potential (CHIP), of which the pathophysiological role in MPN is also not completely understood. PMF, like other MPN, is associated with a chronic hyperinflammatory state characterized by increased concentrations of chemokines, cytokines, such as tumor necrosis factor-alpha (TNF-α) and growth factors. These may contribute to cellular proliferation, clonal evolution and megakaryocyte dysplasia, or may directly stimulate fibrosis through interaction with fibroblasts/mesenchymal cells. Direct immunologic cytotoxicity through release of reactive oxygen/nitrogen species (ROS/RNS) by activated leukocytes (e.g., downstream of TLR sensing of damage associated molecular pattern molecules [DAMP]) may be another pathway resulting in genotoxic stress or megakaryocyte dysplasia. Whether neutrophil engulfment by megakaryocytes (i.e., emperipolesis) directly contributes to megakaryocyte dysplasia remains to be elucidated. Clonal evolution may eventually result in blast transformation or secondary acute myeloid leukemia (sAML). Aberrant megakaryocytes show increased secretion of cytokines such as CXCL8 (interleukin-8 [IL-8]) or transforming growth factor-β (TGF-β) which contribute to the development of PMF.