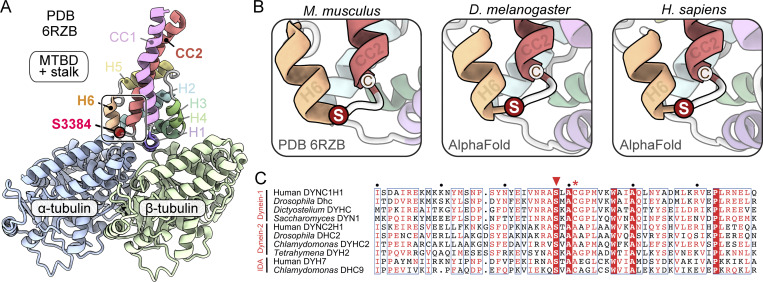
Figure 3.
S3372 has a conserved position in a loop between H6 of the MTBD and CC2 of the stalk. (A) Overview of position of the equivalent residue to S3372 (S3384) in the cryo-EM structure of the mouse MTBD and portion of the stalk in complex with the α/β-tubulin dimer (PDB 6RZB; Lacey et al., 2019). Position of S3384 is highlighted by a red circle. (B) Zoom-ins of regions containing S3384 (S) in 6RZB and equivalent residues in Alphafold2-generated structures of the MTBD and stalk of Drosophila melanogaster Dhc and human DYNC1H1. Neighboring cysteine (C) residues (C3387 mouse; C3375 Drosophila; C3389 human) are also shown. (C) Alignment of sequences from the MTBD and CC2 of the stalk of the indicated dynein family members. White letters on red background, residues present in all sequences; red letters, residues present in ≥50% of sequences; blue boxes, regions with ≥50% conservation; red arrowhead, residues equivalent to S3372 of Drosophila Dhc; red asterisk, residues equivalent to C3375 of Drosophila Dhc. Uniprot accession numbers: human (H. sapiens) DYNC1H1, Q14204; D. melanogaster Dhc, P37276; Dictyostelium discoideum DYHC, P34036; Saccharomyces cerevisiae DYN1, P36022; human DYNC2H1, Q8NCM8; D. melanogaster DHC2, Q0E8P6; Chlamydomonas reinhartii DYHC2, Q9SMH5; Tetrahymena thermophila DYH2, Q5U9X1; human inner dynein arm (IDA) DYH7, Q8WXX0; Chlamydomonas reinhartii IDA DHC9, Q4AC22.