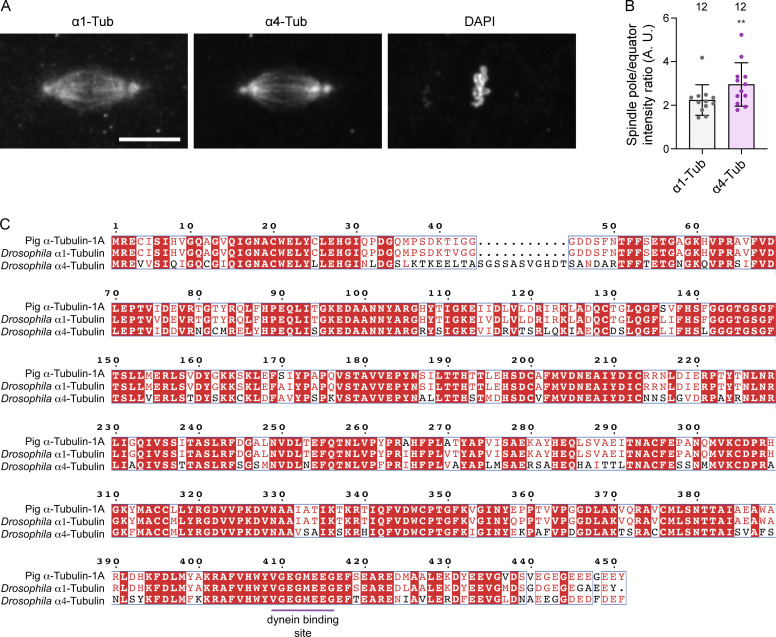
Figure S4.
Assessing a potential α4-tubulin–specific effect of S3372C. (A) Example image of a metaphase spindle in a wild-type embryo stained with antibodies to α1-tubulin (α1-Tub) and α4-tubulin (α4-Tub), as well as DAPI. Scale bar: 10 µm. (B) Quantification of ratio of intensity of α1-Tub and α4-Tub at the pole versus the equator of metaphase spindles. Columns show mean values per spindle; error bars represent SD; circles are values for individual spindles. Number of spindles (randomly selected from five embryos of each genotype) shown above columns. Statistical significance between the ratio values was evaluated with a paired, two-tailed t test. **, P < 0.01. Higher α1-Tub signal at the spindle poles than the equator is presumably due to increased density of microtubules in this region. The observation that α4-Tub has a greater enrichment at the pole than the canonical tubulin isotype indicates preferential accumulation in microtubules at this site. (C) Alignment of protein sequences of mammalian (pig) α-Tub-1A, Drosophila α1-Tub, and Drosophila α4-Tub. White letters on a red background indicate residues present in all sequences; red letters indicate residues present in ≥50% of sequences; blue boxes show regions with ≥50% conservation; magenta horizontal line, region contributing to dynein binding (based on the mouse MTBD-microtubule structure [Lacey et al., 2019]), which is identical in all three proteins. Uniprot accession numbers are: pig (Sus scrofa) α-Tub-1A, P02550; D. melanogaster α1-Tub, P06603; D. melanogaster α4-Tub, P06606.