Scheme 2. Synthetic Route toward “Inverted” Trisubstituted Pyrimidine 12–16, 19–23.
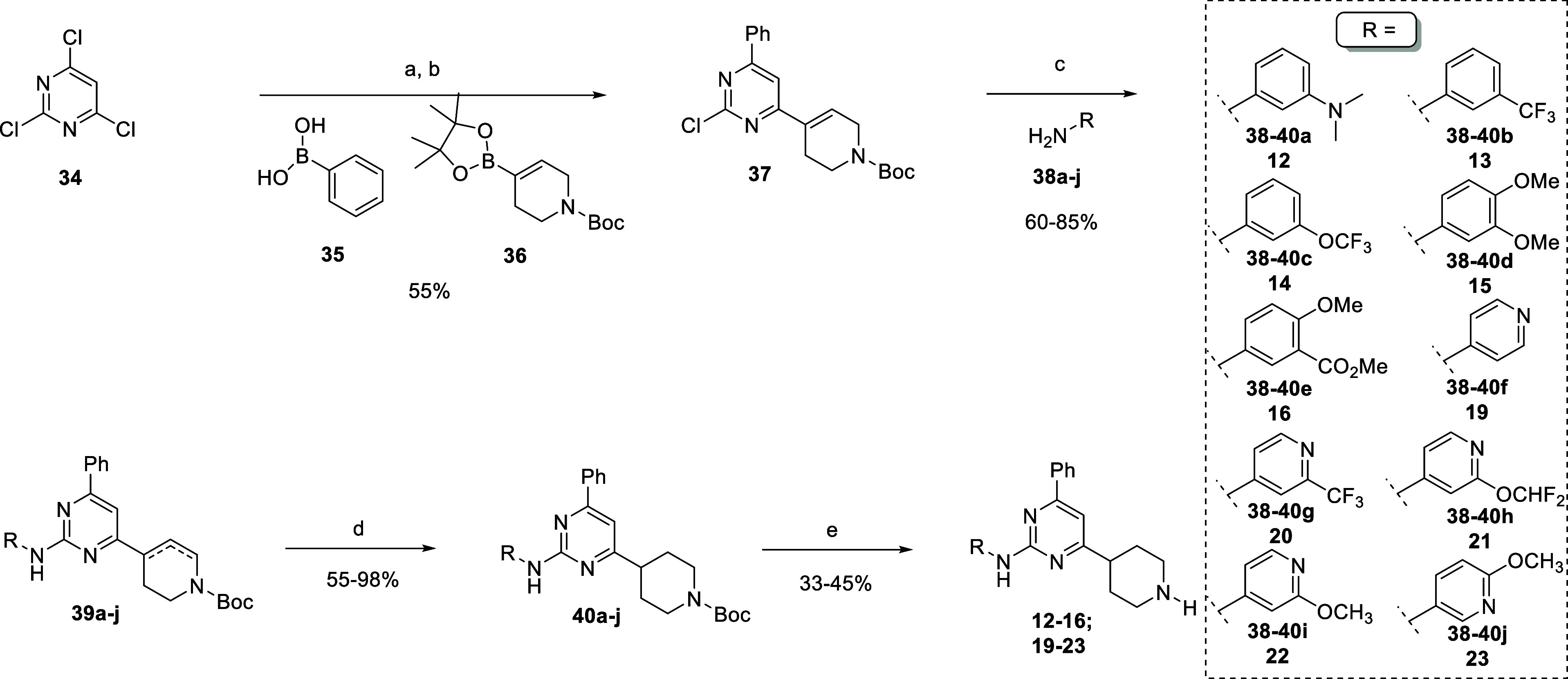
Reagents and conditions: (a) phenylboronic acid 35, PdCl2(dppf)·DCM, K2CO3 (2M)aq, 1,4-dioxane dry, 60 °C, then (b) compound 36, PdCl2(dppf)·DCM, 110 °C; (c) compounds 38a–j, Pd(OAc)2, (±)-BINAP or Xantphos, Cs2CO3, 1,4-dioxane dry, 120 °C; (d) Pd(OH)2/C, NH4COOH, MeOH dry, 80 °C; and (e) HCl (4 M in 1,4-dioxane), 1,4-dioxane dry.
