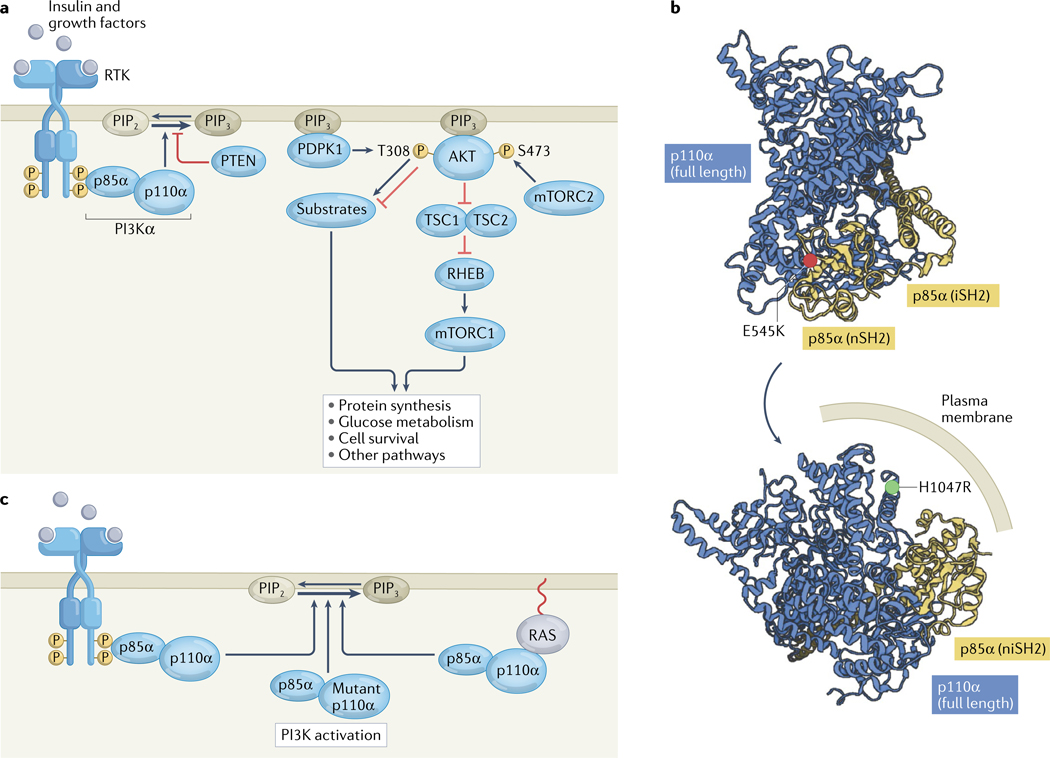
Fig. 1 |. The PI3K pathway in non-malignant cells and cancer.
a | Insulin and other growth factors stimulate receptor tyrosine kinases (RTKs), leading to transautophosphorylation at C-terminal domain tyrosine residues. These phosphotyrosines bind to SH2 domains in the regulatory p85α subunit of phosphatidylinositol 3-kinase-α (PI3Kα), activating the enzymatic p110α subunit and thus PI3Kα activity, catalysing the production of phosphatidylinositol 3,4,5-trisphosphate (PIP3) from phosphatidylinositol 4,5-bisphosphate (PIP2) in the inner layer of the cell membrane. The lipid phosphatase PTEN can reverse this process. PIP3 recruits the serine/threonine kinases PDPK1 and AKT to the plasma membrane. Subsequently, PDPK1 and the mTOR complex 2 (mTORC2) phosphorylate AKT at T308 and S473, respectively. AKT then phosphorylates a host of cellular substrates, including tuberin (also known as TSC2), thus inactivating the TSC complex; suppression of the GTPase-activating protein activity of the TSC complex results in the activation of the GTPase RHEB, which in turn activates mTOR complex 1 (mTORC1), which drives numerous necessary processes in normal physiology and cancer. AKT also regulates cellular processes through various targets other than TSC2. b | Structural depiction of the oncogenic activating p110α E545K and H1047R mutations in the crystal structure of PI3Kα (PDB 4OVU)13. E545K is located at the p110α–p85α binding interface, and H1047R is located in the membrane-binding region. c | In cancer, PI3Kα is often activated through RTK phosphopeptide binding to p85α, p110α mutation or RAS stimulation of p110α. iSH2, inter Src homology 2 domain; niSH2, N-terminal and inter Src homology domains; nSH2, N-terminal Src homology 2 domain.