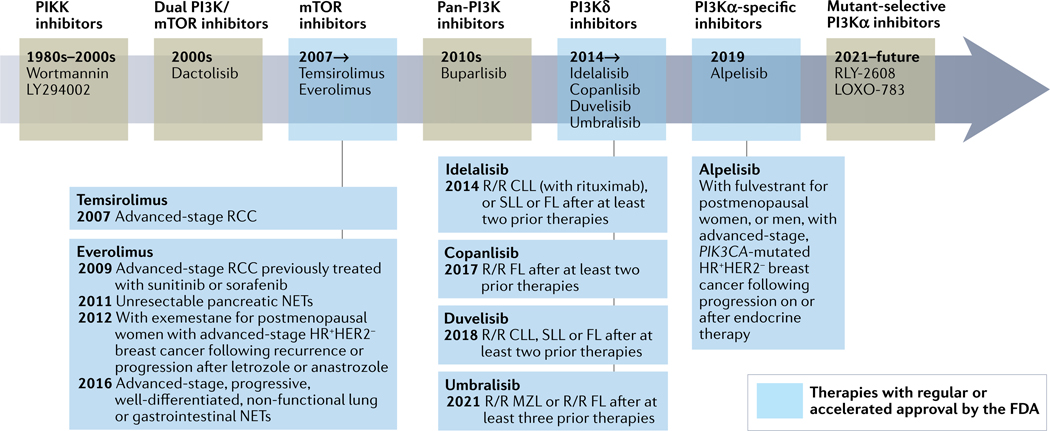
Fig. 2 |. Drugging the PI3K pathway through the decades.
Timeline summarizing phosphatidylinositol 3-kinase (PI3K) pathway inhibitor drug development. FDA-approved drugs and their indications are boxed. CLL, chronic lymphocytic leukaemia; FL, follicular lymphoma; HR, hormone receptor; MZL, marginal zone lymphoma; NETs, neuroendocrine tumours; PIKK, PI3K-related kinase; RCC, renal cell carcinoma; R/R, relapse and/or refractory; SLL, small lymphocytic lymphoma.