Abstract
OBJECTIVES:
To derive systematic review-informed, modified Delphi consensus regarding the management of children on extracorporeal membrane oxygenation (ECMO) undergoing invasive procedures or interventions developed by the Pediatric Anticoagulation on ECMO CollaborativE (PEACE) Consensus Conference.
DATA SOURCES:
A structured literature search was performed using PubMed, EMBASE, and Cochrane Library (CENTRAL) databases from January 1988 to May 2021.
STUDY SELECTION:
ECMO anticoagulation and hemostasis management in the perioperative period and during procedures.
DATA EXTRACTION:
Two authors reviewed all citations independently, with a third independent reviewer resolving any conflicts. Seventeen references were used for data extraction and informed recommendations. Evidence tables were constructed using a standardized data extraction form.
DATA SYNTHESIS:
Risk of bias was assessed using the Quality in Prognosis Studies tool. The evidence was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation system. Forty-eight experts met over 2 years to develop evidence-based recommendations and, when evidence was lacking, expert-based consensus statements for the management of bleeding and thrombotic complications in pediatric ECMO patients. A web-based modified Delphi process was used to build consensus via the Research And Development/University of California Appropriateness Method. Consensus was defined as greater than 80% agreement. Four good practice statements, 7 recommendations, and 18 consensus statements are presented.
CONCLUSIONS:
Although agreement among experts was strong, important future research is required in this population for evidence-informed recommendations.
Keywords: antifibrinolytics, blood transfusions, extracorporeal membrane oxygenation, hemostatic agents, pediatrics, perioperative procedures anticoagulation
Undertaking invasive procedures/interventions for children supported with extracorporeal membrane oxygenation (ECMO) increases the risk of bleeding complications and adds to the complexity of management of anticoagulation for the circuit (1–3). However, procedures may be required to inform diagnosis and treatment options, and intervention may be required to address the respiratory or cardiorespiratory failure precipitating ECMO support (2–6). There are no published evidence-based guidelines to inform the management of anticoagulation, use of antifibrinolytics and hemostatic agents, or transfusion of blood products in the periprocedural period for children on ECMO. The objective of this study was to derive an evidence-informed, modified Delphi consensus regarding the management of periprocedural anticoagulation and hemostasis during pediatric ECMO support.
MATERIALS AND METHODS
Detailed methods and definitions of clinically relevant bleeding are described in the Pediatric Extracorporeal Membrane Oxygenation Anticoagulation Collaborative executive summary (7). Briefly, a structured literature search was performed using PubMed, EMBASE, and Cochrane Library CENTRAL databases from January 1988 to May 2020, with an update in May 2021. Literature on anticoagulation and blood product transfusion management of the periprocedural period and its impact on patient and circuit outcomes were reviewed (Supplemental Methods 1, http://links.lww.com/PCC/C493). Major invasive procedure was defined as an open surgical procedure (e.g., thoracotomy, sternotomy, craniotomy, laparotomy). Minor invasive procedure was defined as any percutaneous procedure (e.g., vascular access, catheter-based intervention, chest tube placement, percutaneous biopsy or drain placement, peritoneal dialysis catheter placement, fasciotomy, and tracheostomy). Minor or routine procedures such as peripheral IV placement, nasogastric tube placement, or urinary catheter placement were not included.
Two authors reviewed all citations independently, with a third independent reviewer resolving any conflicts. Evidence tables were constructed using a standardized data extraction form (7). Risk of bias was assessed using the Quality in Prognosis Studies (QUIPS) tool or the revised Cochrane risk of bias for randomized trials, as appropriate (8–10), and the evidence was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation system (11, 12). A panel of 48 experts met over 2 years to develop evidence-based recommendations and, when evidence was lacking, expert-based consensus statements. The supporting literature was reviewed, and statements were developed using the Evidence to Decision framework, emphasizing the panel’s assessment of risks versus benefits of each proposed statement and a prioritized list of patient outcomes that had been created by a web-based survey of expert panel members (13–15). A web-based modified Delphi process was used to build consensus via the Research And Development/University of California Appropriateness Method. Consensus was defined as greater than 80% agreement. Additional references, not included in the structured literature search, were included in rationale statements to provide context but were not used to derive recommendations or consensus statements.
RESULTS
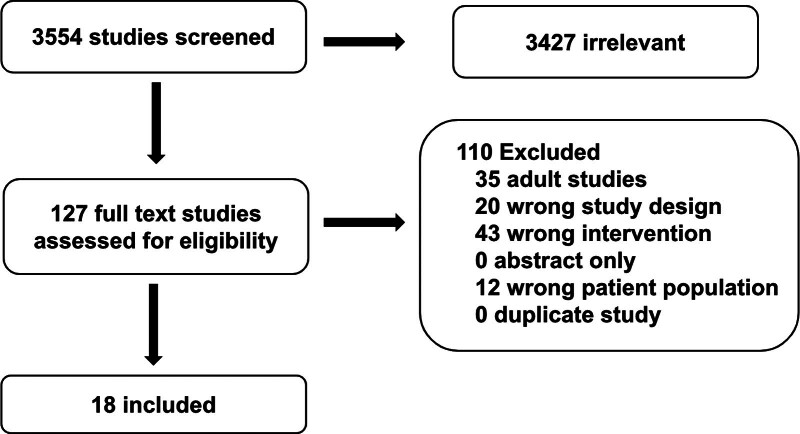
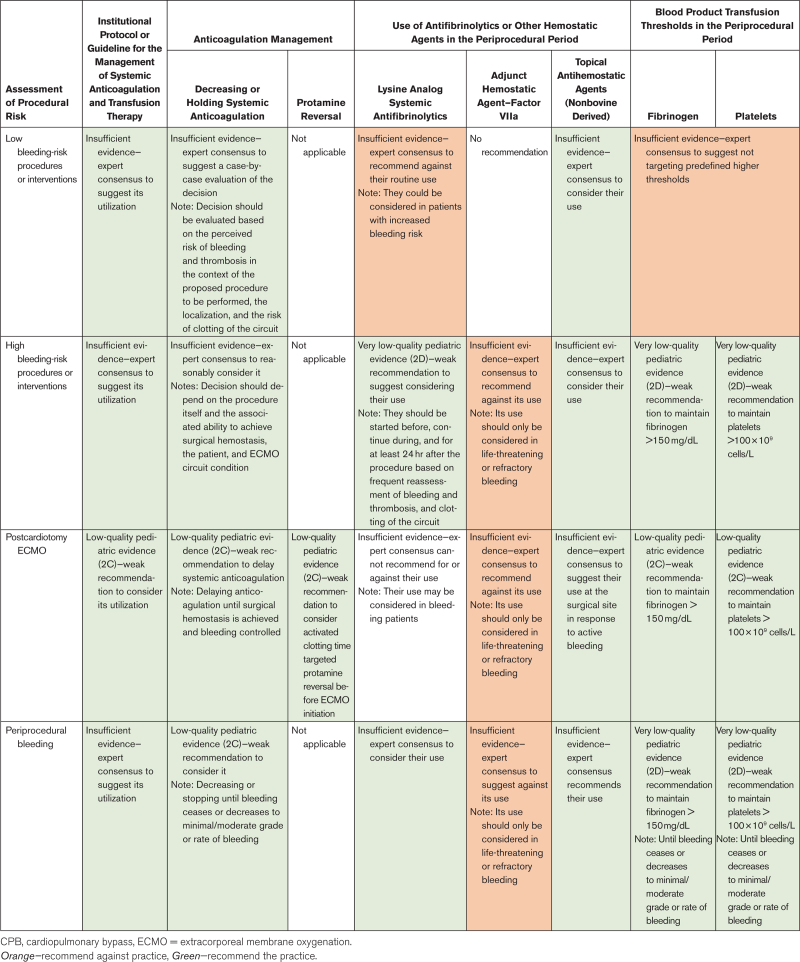
The structured literature search identified 3554 abstracts. Of these, 3427 references were excluded based on the abstract. An additional 110 references were excluded based on the full article review, leaving 17 references that were used for recommendation and consensus statement creation (Fig. 1). The included references are detailed in Supplemental Table 1 (http://links.lww.com/PCC/C493). A summary of the risk of bias assessments is in Supplemental Figure 1 (http://links.lww.com/PCC/C493). Good practice statements, recommendations based on low- or very low-quality pediatric evidence, and consensus statements were developed and reached agreement (> 80%), as summarized in Table 1.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram of studies screened and included in the pediatric extracorporeal membrane oxygenation periprocedural management subgroup.
TABLE 1.
Summary of Interventions During Extracorporeal Membrane Oxygenation Based on the Risk of the Procedure According to Consensus Recommendation
Risk Categories for Periprocedural Bleeding
For a child supported with ECMO, the risk of any proposed procedure/intervention must be weighed against potential benefits in a decision undertaken by the interdisciplinary team in consultation with the parent/guardian. Informed by other professional guidelines addressing procedural bleeding risk, we derived four categories of periprocedural risk of bleeding complications as a framework for these decisions (16–19):
Low bleeding-risk procedures/interventions: Expected to rarely result in hemorrhagic complications, in anatomical regions where bleeding is readily diagnosed and readily controlled.
Postcardiotomy ECMO: Children cannulated to ECMO after cardiotomy or cardiac surgery with cardiopulmonary bypass (CPB) with a high risk of bleeding.
High bleeding-risk procedures (noncardiac): are more likely to result in hemorrhagic complications and/or occur in anatomical regions where bleeding may be difficult to diagnose or treat (e.g., intra-abdominal cavity, lung parenchyma, retroperitoneum) and/or occur in anatomical regions where even minor amounts of bleeding may have devastating consequences (e.g., eye, spinal cord, brain).
Bleeding in the periprocedural period: defined as the 24-hour period after the procedure.
Good Practice Statement
7.1 In ECMO, when a diagnostic or interventional procedure is considered, the benefits and risks, and alternatives of the procedure should be evaluated before deciding to perform an invasive procedure; however, do not postpone the procedure if it impacts diagnosis, treatment and/or prognosis. 89% agreement (n = 46), median 8 (interquartile range [IQR] 7–9).
Procedures on ECMO are frequent, occurring in up to 28.4% of patients studied, depending on inclusion criteria (1, 3). Hemorrhagic (55%) and thrombotic (22%) complications were commonly identified and have been associated with increased mortality (1, 2, 20). Retrospective studies of intercostal catheter insertion or open lung biopsy in children on ECMO documented major bleeding in 20–22%, with up to 11% undergoing thoracotomy for management (21–24). In two registry studies, laparotomy was associated with bleeding in 50% of subjects, with overall high mortality (70%) (24, 25). Studies of children undergoing cardiac catheterization on ECMO demonstrate that 10–20% of children have procedure-related complications. More than half of the patients (68–77%) received diagnoses of residual lesions or unexpected structural or physiologic findings (40%) during catheterization requiring procedural intervention (26–28). Diagnostic procedures during ECMO may result in diagnosis of life-limiting conditions, leading to goal-concordant therapy and redirection of care (23).
Despite the increased risk of bleeding associated with invasive procedures during ECMO support, some children may benefit from the clinical diagnostic information or therapeutic strategy undertaken (4, 29). Retrospective observational studies in children undergoing cardiac catheterization or lung biopsies on ECMO highlight the importance of some procedures for diagnosis, prognosis, and management with acceptable risk of complications (4, 23, 27, 30, 31). Although there is an increased risk of bleeding or other complications associated with invasive procedure/s during ECMO support if that procedure diagnoses and/or treats an underlying condition resulting in a shorter time to successful ECMO decannulation, then the risk/benefit ratio is likely favorable.
Good Practice Statements
7.2 In the ECMO patient with a planned major invasive procedure and/or high bleeding risk of the procedure, identify and optimize underlying coagulation status before the procedure. 89% agreement (n = 44), median 9 (IQR 7–9).
7.3 In the ECMO patient with any risk of bleeding, measure and evaluate blood loss during and after the invasive procedure for at least 24 hours and until the bleeding ceases or decreases to minimal/moderate grade or rate of bleeding. The severity of the bleeding should be determined by the quantity of blood lost, the site of the bleeding, and the consequences of the bleeding on hemodynamics, hemoglobin, and organ dysfunction. 93% agreement (n = 44), median 8 (IQR 7–9).
Hemostatic complications, bleeding, and thrombosis remain the leading causes of morbidity and mortality on ECMO (2, 32). In addition to circuit-surface contact activation, activation of platelets and inflammatory pathways occur in the context of critical illness (32). Management of bleeding includes monitoring anticoagulation and hemostasis, replacement of blood products and coagulation factors, and administering hemostatic agents as needed (33). As procedures or interventions on ECMO are associated with an increased risk of bleeding, we recommend identifying and optimizing underlying coagulation status before invasive procedures. Evaluation for the evolution and potential progression of bleeding in the periprocedural period must be undertaken until bleeding is controlled or ceased. Although there are no consensus definitions for the severity of bleeding during pediatric ECMO, published studies provide a framework including consideration of the volume of bleeding and anatomical location (3, 34–36).
Children Undergoing Low Bleeding-Risk Procedures or Interventions
Consensus Statements
7.4 In ECMO patients undergoing a minor and/or low bleeding-risk procedure, it is reasonable to consider using an institutional protocol or guideline for the management of systemic anticoagulation and transfusion therapy. Consensus panel expertise with weak agreement, 91% agreement (n = 46), median 8 (IQR 7–9).
7.5 In ECMO patients undergoing a minor and/or low bleeding-risk procedure, it is reasonable to consider that the decision to decrease or hold systemic anticoagulation be evaluated case-by-case based on the risk of bleeding and thrombosis in the context of the proposed procedure to be performed, the anatomical location of the invasive procedure, and the risk of clotting of the circuit. Consensus panel expertise with weak agreement, 93% (n = 43), median 8 (IQR 7–9).
7.6 In ECMO patients undergoing a minor and/or low bleeding-risk procedure, it is reasonable to consider the application of nonbovine-derived topical hemostatic agents. Consensus panel expertise with weak agreement, 89% agreement (n = 44), median 8 (IQR 7–9).
7.7 In ECMO patients undergoing a minor and/or low bleeding-risk procedure, we advise against the routine use of lysine analog antifibrinolytic agents (e.g., epsilon aminocaproic acid [EACA], tranexamic acid [TXA]) but they could be considered in patients with concerns for increased risk of bleeding. Consensus panel expertise with strong agreement, 95% agreement (n = 43), median 8 (IQR 7–8).
7.8 In ECMO patients undergoing a minor and/or low bleeding-risk procedure, we suggest against targeting predefined higher thresholds for platelet and fibrinogen transfusions. Consensus panel expertise with weak agreement, 85% agreement (n = 46), median 7.5 (IQR 7–9).
Summary of the Evidence
No data are available to inform the use or selection of a specific protocol or guideline for the management of systemic anticoagulation and transfusion therapy in children undergoing minor or low bleeding-risk procedures on ECMO. Likewise, no pediatric data are available to guide a decrease in or cessation of systemic anticoagulation, use of antifibrinolytic medications, or transfusion thresholds for minor or low bleeding-risk procedures on ECMO.
Data are available to inform the use of nonbovine-derived topical hemostatic agents at cannulation sites in neonates (37). A prospective observational study showed cessation of oozing after the application of fibrin at cannulation site (37). A prospective multicenter randomized controlled trial that compared standard cauterization plus fibrin sealant as a topical hemostatic agent versus standard cauterization alone for cannulation showed decreased bleeding in the fibrin sealant-treated patients (38).
Balance of Benefits Versus Harms
Institutional Protocol
Informed by low-quality pediatric evidence supporting the use of institutional protocols in pediatric anticoagulation postcardiotomy (see 7.9 below), we suggest the use of an institutional protocol for pediatric patients undergoing minor or low bleeding-risk procedures on ECMO. Such protocols may include considerations for how the procedure will be performed (and by whom) to minimize bleeding risk, monitoring for signs of bleeding, and/or processes for interdisciplinary management decisions should bleeding occur. The risk versus benefit of routinely stopping or decreasing systemic anticoagulation for a minor or low bleeding-risk procedure on ECMO is less certain and decisions to alter anticoagulation targets should consider individualized assessments of hemostasis.
Topical Hemostatic Agents
Although there is little evidence of benefit for topical hemostatic agents specific to this population, extrapolated evidence suggests benefit and there is little risk of harm with the use of human-derived topical hemostatic agents. Because of reported cases of immune-mediated coagulopathy with life-threatening bleeding with bovine topical thrombin, we recommend against bovine-derived products (39).
Antifibrinolytic
Because lack of high-quality data to inform lysine analog antifibrinolytic agent use, the associated risk of thrombotic complications, and the low bleeding risk for minor procedures, we suggest against routine prophylactic use of lysine analog antifibrinolytic agents for minor or low bleeding-risk procedures.
Transfusion Thresholds
In the absence of data on transfusion thresholds for platelets or fibrinogen in low bleeding-risk patients, the low rate of associated severe bleeding complications, and the absence of studies evaluating complications of platelet and fibrinogen transfusions in this population, we suggest against prophylactically targeting higher transfusion thresholds in the periprocedural period for minor or low bleeding-risk procedures.
Postcardiotomy ECMO
Recommendations
7.9 In pediatric ECMO patients, before and after CPB, consider using a predefined institutional protocol for the management of systemic anticoagulation and transfusion. Weak recommendation, low-quality pediatric evidence, 91% agreement (n = 43), median 8, IQR 7–9.
7.10 Before starting ECMO during cardiac surgery, consider activated clotting time (ACT)-targeted protamine reversal before ECMO initiation and delaying systemic anticoagulation until after the procedure and until surgical hemostasis is achieved and bleeding is controlled. Weak recommendation, low-quality pediatric evidence, 93% agreement (n = 42), median 7.5, IQR 7–9.
7.11 In ECMO post-CPB, consider maintaining platelets above 100 × 109 cells/L and fibrinogen levels above 150 mg/dL in the periprocedural period. Weak recommendation, low-quality pediatric evidence, 88% agreement (n = 43), median 8, IQR 7–8.
Consensus Statements
7.12 In post-CPB ECMO patients, we cannot suggest for or against the routine use of prophylactic lysine analog antifibrinolytic agents (EACA, TXA). Consensus panel expertise with weak agreement, 91% agreement (n = 43), median 8, IQR 7–8.
7.13 In pediatric ECMO patients post-CPB, lysine analog antifibrinolytic agents (EACA, TXA) may be considered if there is bleeding. Consensus panel expertise with weak agreement, 91% agreement (n = 43), median 8, IQR 7–8.
7.14 In post-CPB ECMO patients, it is reasonable to consider using non-bovine topical hemostatic agents at the surgical site in response to active bleeding as part of a multimodal blood management strategy including surgical hemostasis. Consensus panel expertise with weak agreement, 93% agreement (n = 42), median 8, IQR 7–9.
7.15 In post-CPB ECMO patients—because of the risk of thrombotic complications, we advise against the use of recombinant factor VIIa (rFVIIa) except in the event of life-threatening bleeding refractory to multimodality blood management and resuscitation that addresses factors contributing to bleeding. Consensus panel expertise with strong agreement, 98% agreement (n = 42), median 8, IQR 7–9.
Summary of the Evidence
Low-quality evidence based on observational studies supports the use of an institutional protocol for lowering systemic anticoagulation, maintaining higher fibrinogen and platelet levels, and for the use antifibrinolytics to control bleeding with low rates of circuit thrombosis in ECMO patients after CPB (40–45). In multiple retrospective case-control studies of postcardiotomy ECMO, protocolized use of protamine, maintenance of platelet count greater than or equal to 100 × 109 cells/L, and/or delaying systemic unfractionated heparin (UFH) titration, and/or the use of EACA or TXA were associated with significantly less bleeding (46). Some reports include cessation of UFH therapy for clinically significant bleeding without increased thrombotic complications in postcardiotomy pediatric ECMO (43, 45, 46). Another small study described reduced surgical revision for bleeding with an anticoagulation strategy of continuous antithrombin administration to target antithrombin greater than 100% and low dose (10–20 IU/h) UFH infusion with ACT less than 150 seconds, platelets maintained greater than 100 × 109 cells/L and TXA infusion during ECMO (41).
There is insufficient data to guide routine administration of antifibrinolytic agents during postcardiotomy ECMO in children. In some studies, the lysine analog antifibrinolytics (e.g., EACA or TXA) are part of a predefined institutional protocol (40, 41, 45). One retrospective historical control study showed reduced surgical site bleeding but increased circuit changes in patients at high-bleeding risk including cardiac surgical patients when EACA was administered for 72 hours postprocedure (47).
Limited data informed the consensus statement against the use of rFVIIa in postcardiotomy pediatric ECMO. Two retrospective observational studies in postcardiotomy patients including pediatric ECMO (combined n = 24) reported some evidence of efficacy but an associated increased risk of severe thrombotic complications after rFVIIa administration for severe bleeding (48, 49). Due to the risk of significant circuit thrombosis, if rFVIIa is to be administered, the preparation of a rescue ECMO circuit should be considered.
Balance of Benefits Versus Harms
ECMO support post-CPB in infants and children poses very high bleeding risks with associated morbidity and mortality. As such, efforts to maintain the delicate balance between circuit integrity and patient bleeding risk are paramount. Published institutional protocols have been associated with benefits, although the protocols are highly variable and reported outcomes lack standardization. As such, understanding which protocols or specific elements of protocols may be most efficacious remains a challenge. In the absence of more definitive data, we offer reasonable suggestions meant to balance the risks of severe hemorrhage with risks of patient or circuit thrombosis and risks of transfusion exposure.
Children Undergoing High Bleeding-Risk Procedures (Noncardiac Surgery)
Recommendations
7.16 It is reasonable to consider the use of lysine analog antifibrinolytic agents (EACA, TXA). If administered, we suggest antifibrinolytics be started before the procedure, continued during the procedure, and for at least 24 hours after the procedure based on frequent reassessment of bleeding and thrombosis, and clotting of the circuit. Weak recommendation, very low-quality pediatric evidence, 93% agreement (n = 42), median 7.5, IQR 7–9.
7.17 It is reasonable to consider maintaining platelet thresholds above 100 x 10^9 cells/L and fibrinogen levels above 150 mg/dL in the periprocedural period. Weak recommendation, very low-quality pediatric evidence; 90% agreement (n = 42), median 7.5, IQR 7–9.
Consensus Statements
7.18 It is reasonable to consider prophylactic application of nonbovine-derived topical hemostatic agents at the surgical site. Consensus panel expertise with weak agreement, 90% agreement (n = 42), median 8, IQR 7–9.
7.19 Because of the risk of thrombotic complications, we advise against the use of rFVIIa except in the event of life-threatening bleeding refractory to multimodal blood management and resuscitation that addresses factors contributing to bleeding. Consensus panel expertise with strong agreement, 95% agreement (n = 42), median 8, IQR 7–9.
7.20 It is reasonable to consider using a predefined institutional protocol or guideline for the management of systemic anticoagulation and transfusion therapy in pediatric ECMO during major and/or high bleeding-risk invasive procedures. Consensus panel expertise with weak agreement, 91% agreement (n = 46), median 8, IQR 7–9.
7.21 It is reasonable to consider decreasing or stopping systemic anticoagulation temporarily before the procedure, depending on: the procedure itself; the ability to achieve surgical hemostasis; the patient’s condition; and the state of the ECMO circuit. Consensus panel expertise with weak agreement, 93% agreement (n = 46), median 8, IQR 7–9.
Summary of the Evidence
Antifibrinolytic Agents
Retrospective cohort studies of pediatric ECMO management protocols in the periprocedural period have included reports of less bleeding or blood product transfusions when lysine analog antifibrinolytic agents (e.g., EACA or TXA) are used during open lung biopsy or other surgical procedures (23, 47, 50).
Thresholds for Platelets and Fibrinogen
The formation of clots is largely dependent on the adequacy of platelets and fibrinogen. Thresholds for platelets greater than 100 × 109 cells/L and fibrinogen greater than 150 mg/dL are based on low-quality evidence. Most of the studies are in neonates and targeted thresholds for platelets and fibrinogen are part of broader protocols including thresholds for anticoagulation and/or the use of antifibrinolytics surrounding the procedure (1, 23, 24, 50–53).
Institutional Protocol
There are single-center, retrospective studies of bleeding periprocedure in pediatric ECMO, mainly congenital diaphragmatic hernia (CDH) correction or chest tube placement/lung biopsy, which show reduced bleeding and mortality when a predefined institutional protocol including decrement of systemic anticoagulation, and/or the use of lysine analog antifibrinolytic agents (e.g., EACA, TXA), and/or prophylactic application of non-bovine derived topical hemostatic agents, and/or targeting higher thresholds for platelets and fibrinogen (1, 23, 24, 51–53).
Targets for Anticoagulation
Most of the identified studies that incorporated an institutional protocol consider decrementing or cessation of anticoagulation in the periprocedural period for pediatric ECMO (23, 24, 51–53). Recently, a retrospective case series of protocolized perioperative bivalirudin use for CDH repair on ECMO reported major bleeding in 39% of children, but there were no episodes of reintervention or mortality attributed to hemorrhage and no circuit changes for thrombosis (54).
Balance of Benefits Versus Harms
Similar to considerations for pediatric postcardiotomy ECMO patients, institutional protocols on management of anticoagulation, hemostasis, and transfusion thresholds that balance bleeding risks with risks of circuit or patient thrombosis for pediatric ECMO patients around major invasive or high bleeding-risk procedures are likely beneficial. Due to a lack of evidence and wide variation in institutional protocols, however, identifying a single protocol that maximizes benefit while minimizing harm is challenging and needful for future investigation.
Periprocedural Bleeding
Good Practice Statement
7.22 In ECMO patients who underwent an invasive procedure, early surgical consultation should be sought for procedure-associated bleeding. 96% agreement (n = 46), median 9, IQR 8–9).
Recommendations
7.23 Consider decreasing or stopping systemic anticoagulation temporarily until bleeding ceases or decreases to minimal/moderate grade or rate of bleeding. Weak recommendation, low-quality pediatric evidence, 85% agreement (n = 46), median 8, IQR 7–8.25.
7.24 It is reasonable to consider targeting higher transfusion thresholds by maintaining platelet thresholds above 100 × 109 cells/L and fibrinogen levels above 150 mg/dL until the bleeding ceases or decreases to minimal/moderate grade or rate of bleeding. Weak recommendation, very low-quality pediatric evidence, 93% agreement (n = 42), median 7.5, IQR 7–9.
Consensus Statements
7.25 Consider adoption and use of an institutional protocol for multimodal blood management strategy for periprocedural bleeding which takes into account: 1) the severity of bleeding and/or a bleeding score, 2) close monitoring of the amount of blood losses and the clinical consequences of the bleeding, 3) the need to change the target for systemic anticoagulation and indications for temporarily stopping systemic anticoagulation, 4) indications for the use of antifibrinolytics and/or hemostatic therapies, and 5) the targeting higher concentrations of platelet and fibrinogen levels. Consensus panel expertise with strong agreement, 96% agreement (n= 42), median 8, IQR 7–9.
7.26 In ECMO patients with refractory or severe bleeding associated with a procedure that persists after surgical hemostasis is achieved, it is reasonable to consider consultation with an expert in hemostasis (e.g., intensivist with expertise in ECMO, hematologist, transfusion medicine specialist, hematopathologist, etc.) depending on institutional expertise. Consensus panel expertise with weak agreement, 93% agreement (n = 42), median 8, IQR 7–9.
7.27 It is reasonable to consider the use of lysine analog antifibrinolytic agents (e.g., EACA, TXA) to decrease bleeding as part of a multimodality blood management strategy. Consensus panel expertise with weak agreement, 86% agreement (n = 42), median 7, IQR 7–9.
7.28 In patients with periprocedural bleeding—because of the risk of thrombotic complications, we advise against the use of rFVIIa except in the event of life-threatening bleeding refractory to multimodal blood management and resuscitation that addresses factors contributing to bleeding. Consensus panel expertise with strong agreement, 95% (n = 42), median 8, IQR 7–9.
7.29 In ECMO patients with bleeding associated with an invasive procedure, consider application of nonbovine-derived topical hemostatic agents at the surgical site. Consensus panel expertise with strong agreement, 95% agreement (n = 43), median 8, IQR 7–8.
Summary of the Evidence
Evidence for a predefined institutional protocol, decreasing or ceasing anticoagulation, targeting higher transfusion thresholds for platelets and fibrinogen, and the use of lysine analog antifibrinolytics in high-risk bleeding invasive procedures or patients on ECMO postcardiotomy also apply to patients with periprocedural bleeding (1, 23, 24, 40–42, 46, 48, 50–53).
Balance of Benefits Versus Harms
Given the lack of high-quality clinical evidence to guide the management of periprocedural bleeding in pediatric ECMO patients, it is difficult to assess the balance of benefit versus harm for individual interventions. Because bleeding and associated transfusion requirements carry significant risk, efforts to control bleeding as quickly as possible—including early consideration for surgical hemostasis—are vital. The presented consensus statements are informed by studies of protocolized management of infants and children on ECMO support status post-CPB or congenital diaphragmatic surgery and are intended to balance the risks of prolonged bleeding with risks of transfusion exposure and risks of circuit or patient thrombosis that could arise from bleeding management.
CONCLUSIONS
Procedures or surgical interventions are sometimes required for children supported with ECMO. Limited evidence informs the safety and consequences of such procedures in this population. Balancing risks of bleeding associated with anticoagulation and coagulopathy during pediatric ECMO is even more complicated in the periprocedural period. Decreasing anticoagulation targets, the use of systemic antifibrinolytics or adjunct hemostatic agents, and targeting higher transfusion thresholds for blood products may decrease bleeding events at the risk of thrombosis or circuit clotting. Strong evidence is lacking in this population and further research is needed to stratify the risk of such procedures, improve anticoagulation management, administration of antifibrinolytics, and determine thresholds for transfusions for blood products.
ACKNOWLEDGMENTS
We thank all members of the Pediatric Extracorporeal Membrane Oxygenation Anticoagulation CollaborativE (PEACE) for their support, especially during the COVID-19 pandemic. The authors acknowledge the important contributions of Dr. M. Patricia Massicotte to the design and execution of the PEACE project. In addition, we thank AABB, the American Society of Extracorporeal Therapists, the American Pediatric Surgical Association, the Children’s Hospital Neonatal Consortium, the Collaborative Pediatric Critical Care Research Network, the European Society for Pediatric and Neonatal Intensive Care, the Extracorporeal Life Support Organization, the International Society of Blood Transfusion, Pediatric Cardiac Critical Care Consortium, Pediatric Cardiac Intensive Care Society, the Society for Critical Care Medicine (Pediatric Section and Clinical Pharmacy and Pharmacology Section) and the Society of Thoracic Surgeons.
Supplementary Material
Footnotes
Pediatric Extracorporeal Membrane Oxygenation Anticoagulation CollaborativE (PEACE) members are listed in Appendix 1 (http://links.lww.com/PCC/C493).
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/pccmjournal).
This work was supported by funding from the National Institutes of Health/the National Institute of Child Health and Human Development (R13HD104432 Pediatric Extracorporeal Membrane Oxygenation [ECMO] Anticoagulation CollaborativE); an unrestricted grant from the Extracorporeal Life Support Organization; and contributions from the Abigail Wexner Research Institute at Nationwide Children’s Hospital, Department of ECMO and Advanced Technologies, Children’s Healthcare of Atlanta, and Department of Cardiology, Boston Children’s Hospital.
The Executive Committee (Drs. Alexander, Muszynski, Bembea, Cheifetz, Steiner, and Barbaro) served as arbitrators for conflict of interest management. Dr. Alexander’s institution received funding from Novartis (Prospective Trial to Assess the Angiotensin ReceptorBlocker Neprilysin Inhibitor LCZ696 Versus Angiotensin-Converting Enzyme Inhibitor for the Medical Treatment of Pediatric HF [PANORAMA-HF]). Drs. Muszynski and Alexander’s institutions received funding from the National Institutes of Health (NIH); they received support for article research from the NIH. Dr. Alexander’s institution received funding from the Extracorporeal Life Support Organization and Novartis. Dr. Emani received funding from Cheisi Pharma and Cellvie Bio. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Contributor Information
Collaborators: Peta M.A. Alexander, Melania M. Bembea, Katherine Cashen, Ira M. Cheifetz, Heidi Dalton, Adam S. Himebauch, Oliver Karam, Katie M. Moynihan, FRACP FCIC, Marianne E. Nellis, Caroline Ozment, Lakshmi Raman, Natalie E. Rintoul, Ahmed S. Said, Arun Saini, Marie E. Steiner, Ravi R. Thiagarajan, Kevin Watt, Ariane Willems, Nicole D. Zantek, Ryan P. Barbaro, Katherine Steffen, Adam M. Vogel, Christopher Almond, Marc M. Anders, Gail M. Annich, Leonardo R. Brandão, Wayne Chandler, Megan Delaney, Robert DiGeronimo, Sitaram Emani, Samir K.l Gadepalli, Alejandro V. Garcia, Bereketeab Haileselassie, Adam S. Himebauch, Robert Hyslop, Martin C.J. Kneyber, Lisa Baumann Kreuziger, Jennifer Le, Laura Loftis, Ali B.V. McMichael, D. Michael McMullan, Paul Monagle, Kathleen Nicol, Matthew L. Paden, Jason Patregnani, John R. Priest, Leslie Raffini, Lindsay M. Ryerson, Steven R. Sloan, Jun Teruya, Andrew R. Yates, Alison Gehred, Elizabeth Lyman, and Jennifer A. Muszynski
REFERENCES
- 1.Nagaraj HS, Mitchell KA, Fallat ME, et al. : Surgical complications and procedures in neonates on extracorporeal membrane oxygenation. J Pediatr Surg. 1992; 27:1106–1109; discussion 1109 [DOI] [PubMed] [Google Scholar]
- 2.Barbaro RP, Paden ML, Guner YS, et al. ; ELSO member centers: Pediatric extracorporeal life support Organization Registry International Report 2016. ASAIO J. 2017; 63:456–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalton HJ, Reeder R, Garcia-Filion P, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network: Factors associated with bleeding and thrombosis in children receiving extracorporeal membrane oxygenation. Am J Respir Crit Care Med. 2017; 196:762–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howard TS, Kalish BT, Wigmore D, et al. : Association of extracorporeal membrane oxygenation support adequacy and residual lesions with outcomes in neonates supported after cardiac surgery. Pediatr Crit Care Med. 2016; 17:1045–1054 [DOI] [PubMed] [Google Scholar]
- 5.Dao DT, Burgos CM, Harting MT, et al. : Surgical repair of congenital diaphragmatic hernia after extracorporeal membrane oxygenation cannulation: Early repair improves survival. Ann Surg. 2021; 274:186–194 [DOI] [PubMed] [Google Scholar]
- 6.Sengupta A, Gauvreau K, Kaza A, et al. : Influence of intraoperative residual lesions and timing of extracorporeal membrane oxygenation on outcomes following first-stage palliation of single-ventricle heart disease. J Thorac Cardiovasc Surg. 2023; 165:2181–2192.e2 [DOI] [PubMed] [Google Scholar]
- 7.Alexander PMA, Alexander PMA, Bembea M, Cashen K, et al. ; Pediatric Extracorporeal Membrane Oxygenation (ECMO) Anticoagulation CollaborativE (PEACE), in collaboration with the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network, the Pediatric Critical Care Blood Research Network (BloodNet), and the Pediatric ECMO subgroup of PALISI and the Extracorporeal Life Support Organization (PediECMO): Executive summary: The Pediatric Extracorporeal Membrane Oxygenation Anticoagulation CollaborativE Consensus Conference. Pediatr Crit Care Med. 2024; 25:643–675 [Google Scholar]
- 8.Sterne JAC, Savovic J, Page MJ, et al. : RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019; 366:l4898. [DOI] [PubMed] [Google Scholar]
- 9.Higgins JP, Sterne JA, Savovic J, et al. : A revised tool for assessing risk of bias in randomized trials. Cochrane Methods. Chandler J, McKenzie J, Boutron I, et al. (Eds). Cochrane Database of Systematic Reviews; 2016; 10 (Suppl 1) [Google Scholar]
- 10.Hayden JA, van der Windt DA, Cartwright JL, et al. : Assessing bias in studies of prognostic factors. Ann Intern Med. 2013; 158:280–286 [DOI] [PubMed] [Google Scholar]
- 11.Balshem H, Helfand M, Schunemann HJ, et al. : GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011; 64:401–406 [DOI] [PubMed] [Google Scholar]
- 12.Neumann I, Santesso N, Akl EA, et al. : A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016; 72:45–55 [DOI] [PubMed] [Google Scholar]
- 13.Neumann I, Brignardello-Petersen R, Wiercioch W, et al. : The GRADE evidence-to-decision framework: A report of its testing and application in 15 international guideline panels. Implement Sci. 2016; 11:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alonso-Coello P, Oxman AD, Moberg J, et al. ; GRADE Working Group: GRADE Evidence to Decision (EtD) frameworks: A systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016; 353:i2089. [DOI] [PubMed] [Google Scholar]
- 15.Alonso-Coello P, Schunemann HJ, Moberg J, et al. ; GRADE Working Group: GRADE Evidence to Decision (EtD) frameworks: A systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016; 353:i2016. [DOI] [PubMed] [Google Scholar]
- 16.Nichols WL, Hultin MB, James AH, et al. : The Diagnosis, Evaluation and Management of von Willebrand Disease. In: U.S. Department of Health and Human Services, National Heart, Lung and Blood Institute, (Ed). Bethesda, MD, Department of Health and Human Servies, NIH Publication No. 08-5832; 2007 [Google Scholar]
- 17.Malloy PC, Grassi CJ, Kundu S, et al. ; Standards of Practice Committee with Cardiovascular and Interventional Radiological Society of Europe (CIRSE) Endorsement: Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2009; 20:S240–S249 [DOI] [PubMed] [Google Scholar]
- 18.Patel IJ, Rahim S, Davidson JC, et al. : Society of Interventional Radiology Consensus Guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions—part ii: Recommendations: Endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol. 2019; 30:1168–1184.e1 [DOI] [PubMed] [Google Scholar]
- 19.Douketis JD, Spyropoulos AC, Duncan J, et al. : Perioperative management of patients with atrial fibrillation receiving a direct oral anticoagulant. JAMA Intern Med. 2019; 179:1469–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reed RC, Rutledge JC: Laboratory and clinical predictors of thrombosis and hemorrhage in 29 pediatric extracorporeal membrane oxygenation nonsurvivors. Pediatr Dev Pathol. 2010; 13:385–392 [DOI] [PubMed] [Google Scholar]
- 21.Jackson HT, Longshore S, Feldman J, et al. : Chest tube placement in children during extracorporeal membrane oxygenation (ECMO). J Pediatr Surg. 2014; 49:51–53; discussion 53 [DOI] [PubMed] [Google Scholar]
- 22.Tashiro J, Perez EA, Lasko DS, et al. : Post-ECMO chest tube placement: A propensity score-matched survival analysis. J Pediatr Surg. 2015; 50:793–797 [DOI] [PubMed] [Google Scholar]
- 23.Houmes RJ, Ten Kate CA, Wildschut ED, et al. : Risk and relevance of open lung biopsy in pediatric ECMO patients: The Dutch experience. J Pediatr Surg. 2017; 52:405–409 [DOI] [PubMed] [Google Scholar]
- 24.Phillips MR, Khoury AL, Stephenson BJ, et al. : Outcomes of pediatric patients with abdominal sepsis requiring surgery and extracorporeal membrane oxygenation using the Extracorporeal Life Support Organization database. Am Surg. 2015; 81:245–251 [PMC free article] [PubMed] [Google Scholar]
- 25.Barry WE, Castle SL, Golden J, et al. : Laparotomy complications on extracorporeal life support: Surgical site bleeding does not increase mortality. J Pediatr Surg. 2019; 54:1736–1739 [DOI] [PubMed] [Google Scholar]
- 26.desJardins SE, Crowley DC, Beekman RH, et al. : Utility of cardiac catheterization in pediatric cardiac patients on ECMO. Catheter Cardiovasc Interv. 1999; 46:62–67 [DOI] [PubMed] [Google Scholar]
- 27.Booth KL, Roth SJ, Perry SB, et al. : Cardiac catheterization of patients supported by extracorporeal membrane oxygenation. J Am Coll Cardiol. 2002; 40:1681–1686 [DOI] [PubMed] [Google Scholar]
- 28.Kato A, Lo Rito M, Lee KJ, et al. : Impacts of early cardiac catheterization for children with congenital heart disease supported by extracorporeal membrane oxygenation. Catheter Cardiovasc Interv. 2017; 89:898–905 [DOI] [PubMed] [Google Scholar]
- 29.Thomas J, Kostousov V, Teruya J: Bleeding and thrombotic complications in the use of extracorporeal membrane oxygenation. Semin Thromb Hemost. 2018; 44:20–29 [DOI] [PubMed] [Google Scholar]
- 30.Callahan R, Trucco SM, Wearden PD, et al. : Outcomes of pediatric patients undergoing cardiac catheterization while on extracorporeal membrane oxygenation. Pediatr Cardiol. 2015; 36:625–632 [DOI] [PubMed] [Google Scholar]
- 31.Guzeltas A, Kasar T, Tanidir IC, et al. : Cardiac catheterization procedures in pediatric patients undergoing extracorporeal membrane oxygenation cardiac catheterization, ECMO. Anatol J Cardiol. 2017; 18:425–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy DA, Hockings LE, Andrews RK, et al. : Extracorporeal membrane oxygenation-hemostatic complications. Transfus Med Rev. 2015; 29:90–101 [DOI] [PubMed] [Google Scholar]
- 33.Penk JS, Reddy S, Polito A, et al. : Bleeding and thrombosis with pediatric extracorporeal life support: A roadmap for management, research, and the future from the pediatric cardiac intensive care society: Part 2. Pediatr Crit Care Med. 2019; 20:1034–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nellis ME, Tucci M, Lacroix J, et al. ; Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network; and the Pediatric Critical Care Blood Research Network (BloodNet): Bleeding Assessment Scale in Critically Ill Children (BASIC): Physician-driven diagnostic criteria for bleeding severity. Crit Care Med. 2019; 47:1766–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Halloran CP, Andren KG, Mecklosky J, et al. : Mortality and factors associated with hemorrhage during pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2020; 21:75–81 [DOI] [PubMed] [Google Scholar]
- 36.Levy JH, Faraoni D, Almond CS, et al. : Consensus statement: hemostasis trial outcomes in cardiac surgery and mechanical support. Ann Thorac Surg. 2022; 113:1026–1035 [DOI] [PubMed] [Google Scholar]
- 37.Moront MG, Katz NM, O’Connell J, et al. : The use of topical fibrin glue at cannulation sites in neonates. Surg Gynecol Obstet. 1988; 166:358–359 [PubMed] [Google Scholar]
- 38.Atkinson JB, Gomperts ED, Kang R, et al. : Prospective, randomized evaluation of the efficacy of fibrin sealant as a topical hemostatic agent at the cannulation site in neonates undergoing extracorporeal membrane oxygenation. Am J Surg. 1997; 173:479–484 [DOI] [PubMed] [Google Scholar]
- 39.Rodgers GM: Immune-mediated coagulopathy associated with topical bovine thrombin: Review of the pediatric literature. J Pediatr Hematol Oncol. 2011; 33:86–88 [DOI] [PubMed] [Google Scholar]
- 40.ElMahrouk AF, Ismail MF, Hamouda T, et al. : Extracorporeal membrane oxygenation in postcardiotomy pediatric patients—15 years of experience outside Europe and North America. Thorac Cardiovasc Surg. 2019; 67:28–36 [DOI] [PubMed] [Google Scholar]
- 41.Agati S, Ciccarello G, Salvo D, et al. : Use of a novel anticoagulation strategy during ECMO in a pediatric population: Single-center experience. ASAIO J. 2006; 52:513–516 [DOI] [PubMed] [Google Scholar]
- 42.Muensterer OJ, Laney D, Georgeson KE: Survival time of ECMO circuits on and off bleeding protocol: Is there a higher risk of circuit clotting? Eur J Pediatr Surg. 2011; 21:30–32 [DOI] [PubMed] [Google Scholar]
- 43.O’Meara LC, Alten JA, Goldberg KG, et al. : Anti-xa directed protocol for anticoagulation management in children supported with extracorporeal membrane oxygenation. ASAIO J. 2015; 61:339–344 [DOI] [PubMed] [Google Scholar]
- 44.Yang F, Hou D, Wang J, et al. : Vascular complications in adult postcardiotomy cardiogenic shock patients receiving venoarterial extracorporeal membrane oxygenation. Ann Intensive Care. 2018; 8:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.von Stumm M, Subbotina I, Biermann D, et al. : Impact of delayed systemic heparinization on postoperative bleeding and thromboembolism during post-cardiotomy extracorporeal membrane oxygenation in neonates. Perfusion. 2020; 35:626–632 [DOI] [PubMed] [Google Scholar]
- 46.Nardell K, Annich GM, Hirsch JC, et al. : Risk factors for bleeding in pediatric post-cardiotomy patients requiring ECLS. Perfusion. 2009; 24:191–197 [DOI] [PubMed] [Google Scholar]
- 47.Downard CD, Betit P, Chang RW, et al. : Impact of AMICAR on hemorrhagic complications of ECMO: A ten-year review. J Pediatr Surg. 2003; 38:1212–1216 [DOI] [PubMed] [Google Scholar]
- 48.Agarwal HS, Bennett JE, Churchwell KB, et al. : Recombinant factor seven therapy for postoperative bleeding in neonatal and pediatric cardiac surgery. Ann Thorac Surg. 2007; 84:161–168 [DOI] [PubMed] [Google Scholar]
- 49.Kurkluoglu M, Engle AM, Costello JP, et al. : Single center experience on dosing and adverse events of recombinant factor seven use for bleeding after congenital heart surgery. J Saudi Heart Assoc. 2015; 27:18–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van der Staak FH, de Haan AF, Geven WB, et al. : Surgical repair of congenital diaphragmatic hernia during extracorporeal membrane oxygenation: Hemorrhagic complications and the effect of tranexamic acid. J Pediatr Surg. 1997; 32:594–599 [DOI] [PubMed] [Google Scholar]
- 51.Dassinger MS, Copeland DR, Gossett J, et al. ; Congenital Diaphragmatic Hernia Study Group: Early repair of congenital diaphragmatic hernia on extracorporeal membrane oxygenation. J Pediatr Surg. 2010; 45:693–697 [DOI] [PubMed] [Google Scholar]
- 52.Keijzer R, Wilschut DE, Houmes RJ, et al. : Congenital diaphragmatic hernia: To repair on or off extracorporeal membrane oxygenation? J Pediatr Surg. 2012; 47:631–636 [DOI] [PubMed] [Google Scholar]
- 53.Fallon SC, Cass DL, Olutoye OO, et al. : Repair of congenital diaphragmatic hernias on extracorporeal membrane oxygenation (ECMO): Does early repair improve patient survival? J Pediatr Surg. 2013; 48:1172–1176 [DOI] [PubMed] [Google Scholar]
- 54.Snyder CW, Goldenberg NA, Nguyen ATH, et al. : A perioperative bivalirudin anticoagulation protocol for neonates with congenital diaphragmatic hernia on extracorporeal membrane oxygenation. Thromb Res. 2020; 193:198–203 [DOI] [PubMed] [Google Scholar]