Abstract
Objective:
People with HIV (PWH) have high risk of liver fibrosis. We investigated the effect of weight gain and metabolic dysfunction-associated steatotic liver disease (MASLD) on liver fibrosis dynamics.
Design:
Multicenter cohort study.
Methods:
Fibrosis progression was defined as development of significant fibrosis [liver stiffness measurement (LSM) ≥8 kPa], or transition to cirrhosis (LSM ≥13 kPa), for those with significant fibrosis at baseline. Fibrosis regression was defined as transition to LSM less than 8 kPa, or to LSM less than 13 kPa for those with cirrhosis at baseline. MASLD was defined as hepatic steatosis (controlled attenuation parameter >248 dB/m) with at least one metabolic abnormality. A continuous-time multistate Markov model was used to describe transitions across fibrosis states.
Results:
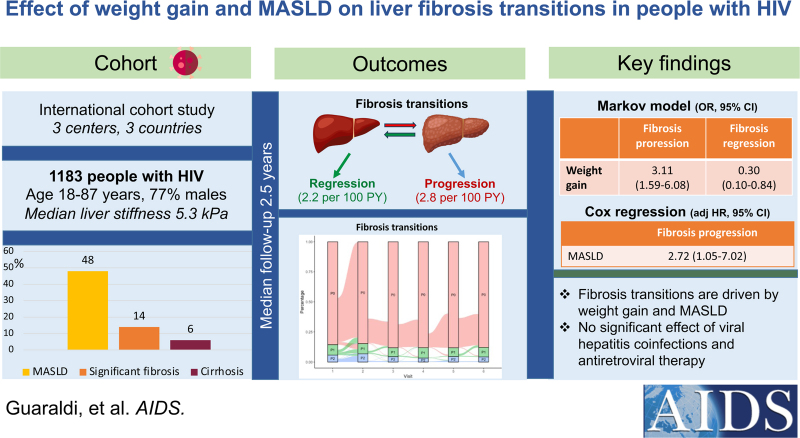
Among 1183 PWH included from three centers (25.2% with viral hepatitis coinfection), baseline prevalence of significant fibrosis and MASLD was 14.4 and 46.8%, respectively. During a median follow-up of 2.5 years (interquartile range 1.9–3.5), the incidence rate of fibrosis progression and regression was 2.8 [95% confidence interval (CI) 2.3–3.4] and 2.2 (95% CI 1.9–2.6) per 100 person-years, respectively. In Markov model, weight gain increased the odds of fibrosis progression [odds ratio (OR) 3.11, 95% CI 1.59–6.08], whereas weight gain (OR 0.30, 95% CI 0.10–0.84) and male sex (OR 0.32, 95% CI 0.14–0.75) decreased the odds of fibrosis regression. On multivariable Cox regression analysis, predictors of fibrosis progression were weight gain [adjusted hazard ratio (aHR) 3.12, 95% CI 1.41–6.90] and MASLD (aHR 2.72, 95% CI 1.05–7.02).
Conclusion:
Fibrosis transitions are driven by metabolic health variables in PWH, independently of viral hepatitis coinfection and antiretroviral class therapy.
Keywords: antiretroviral therapy, controlled attenuation parameter, liver fibrosis, steatotic liver disease, transient elastography, viral hepatitis, weight gain
Introduction
In the era of combination antiretroviral therapy (ART), liver diseases have become a leading cause of morbidity and mortality in people with HIV (PWH) [1]. While co-infection with hepatitis B (HBV) and C virus (HCV) has long determined this trend, hepatic steatosis has recently emerged as the most frequent liver disease in people aging with HIV [2]. Independently of the underlying cause, the natural history of any chronic liver disease is driven by the formation of liver fibrosis. The accumulation of fibrosis eventually leads to progressive distortion of the hepatic architecture, that is the hallmark of the evolution to cirrhosis. The staging of liver fibrosis is essential for risk stratification and prediction of liver-related complications and all-cause mortality [3]. In PWH, significant liver fibrosis seems particularly frequent, with a recent meta-analysis collocating its prevalence at 12% in those without viral hepatitis coinfection [4]. This excess is likely due to a more complex pathogenesis, including frequent metabolic comorbidities, persistent immune activation, hepatocytopathic effect of HIV itself, long-term use of ART, past exposure to hepatotoxic dideoxynucleoside drugs (didanosine and stavudine), hepatic steatosis and ART-associated weight gain [5,6]. In consideration of this burden, guidelines from the European AIDS Clinical Society recommend case-finding of liver fibrosis in PWH with metabolic conditions or persistently elevated transaminases [7].
The initiation of ART may result in weight gain and lipid changes among PWH. Integrase strand transfer inhibitors (INSTI)-based regimens are highly efficacious for viral suppression. However, they may cause more weight gain and treatment emergent obesity than non-INSTI-based regimens and may increase the risk of weight-related comorbidities, including hepatic steatosis [8–10]. In June 2023, an international consensus panel introduced steatotic liver disease (SLD) as an umbrella term encompassing the various causes of hepatic steatosis [11]. Metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as nonalcoholic fatty liver disease (NAFLD), has been defined as evidence of hepatic steatosis in more than 5% of hepatocytes with at least one cardiometabolic risk factor, in the absence of excessive alcohol intake or other known causes of SLD. MASLD provides a positive rather than negative diagnosis, appropriately assigns a metabolic basis for this liver disease, avoids any potentially stigmatizing term, and excludes alcohol abuse [11]. The clinical and histological spectrum of MASLD ranges from simple steatosis to metabolic dysfunction-associated steatohepatitis (MASH), a necro-inflammatory condition eventually leading to liver fibrosis and cirrhosis [12].
To date, no studies have evaluated the role of weight gain and of the new MASLD definition on liver fibrosis dynamics in PWH, as well as the contribution of ART and viral hepatitis coinfection. In an international cohort collaboration of PWH, we aimed to determine the effect of weight gain, MASLD, viral hepatitis coinfection and antiretroviral class exposure on liver fibrosis progression and regression.
Patients and methods
Study design and population
We conducted a retrospective analysis of three cohorts of PWH with or without hepatitis coinfection undergoing screening for liver fibrosis: the LIVEr disease in HIV (LIVEHIV) at the McGill University Health Centre (MUHC), Modena HIV Metabolic Clinic (MHMC), and the University Hospital Bonn Cohort. Between January 2015 and December 2021, we enrolled consecutive patients aged at least 18 years with confirmed HIV infection on ART, availability of liver stiffness measurement (LSM) and controlled attenuation parameter (CAP) by transient elastography, and relevant clinical and biochemical parameters. Exclusion criteria were: less than two consecutive transient elastography examinations; contraindications (pregnancy, pacemaker insertion) and failure or unreliable measurement of transient elastography examination; significant alcohol intake (>20 g/day for women and >30 g/day for men). The combined cohort of 1183 PWH included 122 (10.3%) patients from the LIVEHIV, 691 (58.4%) from the MHMC, 370 (31.3%) from the Bonn University Hospital Cohort.
Ethics
All participants provided informed written consent. The Research Ethics Board of the Research Institute of MUHC (study code 14-182-BMD), of MHMC (study code 254/12) and of the Bonn University Hospital (279/14, 2014, 2016 and 2019) approved the study. The study was conducted according to the Declaration of Helsinki and the manuscript was prepared according to the STROBE Statement-checklist of items.
Clinical and biological parameters
We collected data within 3 months from the transient elastography examination, namely demographic information, time since HIV diagnosis (defined as the interval between the date of patients’ first positive HIV test and the date of the visit), ART class [nonnucleoside reverse transcriptase inhibitors (NNRTI), protease inhibitors, INSTI, tenofovir alafenamide (TAF), nucleoside reverse transcriptase inhibitors (NRTI), including stavudine and didanosine], BMI, liver serum biomarkers, lipid profile, hematological and immune-virological parameters. Undetectable viral load was defined as HIV viral load less than 50 copies/ml. Weight gain was defined as an increase in BMI greater than 5% from the baseline until the end of follow-up. Under/normal weight, overweight, and obesity were defined by BMI values of less than 25, 25–29, and at least 30 kg/m2, respectively. Cardiovascular events were any among prior myocardial infarction, revascularization, angina, stroke, or cardiovascular disease equivalent, such as peripheral arterial disease. MASLD was defined as the presence of hepatic steatosis, defined as CAP at least 248 dB/m [13,14], plus at least one of the following criteria:
-
(1)
BMI at least 25 kg/m2;
-
(2)
Previous diagnosis or treatment for type 2 diabetes;
-
(3)
Blood pressure at least 130/85 mmHg or treatment for hypertension;
-
(4)
Triglycerides greater than 1.69 mmol/l or lipid lowering therapy;
-
(5)
HDL-C less than 1.03 mmol/l (men) or less than 1.30 mmol/l (women) or lipid-lowering therapy [11].
The fibrosis biomarker Fibrosis-4 index (FIB-4) was computed. A cut-off of FIB-4 at least 2.67 was used to identify significant liver fibrosis [15].
Transient elastography examination
Transient elastography examinations (Echosens, Paris, France) were performed on a 4 h fasting patient by maximum two operators at each site using standard quality criteria [16]. Patients were initially assessed with the standard M probe; the XL probe was used in case of failure of the M probe and in patients with BMI at least 30 kg/m2 or a thicker layer of subcutaneous fat. A reliable LSM was defined as at least 10 valid readings with an interquartile range 30% or less of the median value. Significant liver fibrosis (stage F2–4 out of 4) was defined as LSM at least 8 kPa. Cirrhosis (stage F4 out of 4) was defined as LSM at least 13 kPa [17,18].
Outcome measures
The primary study outcomes were: fibrosis progression, defined as development of significant liver fibrosis, or transition to cirrhosis for those with LSM at least 8 but less than 13 kPa at baseline; fibrosis regression, defined as transition to LSM less than 8 kPa for those with significant liver fibrosis, or to LSM less than 13 kPa for those with cirrhosis at baseline. The duration of follow-up was calculated from the first reliable LSM to the date of the last LSM, until December 2021.
Statistical methods
Baseline (time zero) was set as the first visit after 1 January 2015, when LSM was determined. The follow-up was set at the last visit in which LSM was evaluated. Incidence rates of liver fibrosis progression and regression were estimated by dividing the number of participants developing the outcome by the number of person-years of follow-up. A continuous time multistate Markov model reporting odds ratio with 95% confidence interval was used to describe the process in which a study patient moved through a series of states allowing joint analysis of care length, incidence fibrosis progression or reversion. The probabilities of switch from one state to another were modeled according to an exponential distribution for time-to-event data, considering censored follow-up times. The events were the transitions between the states, considered as fibrosis progression and regression. Analyses were performed using minimum two and maximum six assessments for LSM. MASLD was not included in Markov models because of a significant proportion of missing data during follow-up period. A Cox proportional hazard model was applied to test the effect of baseline MASLD and chronic HBV and HCV coinfection on liver fibrosis progression. As key confounders, the following were identified: age, sex, BMI gain greater than 5%, HIV-related variables (time since HIV diagnosis, nadir CD4+ cell count) and antiretroviral regimens (current exposure to INSTI, TAF, protease inhibitor, and NNRTI). The effect on the outcome was expressed as hazard ratio with 95% CI. A complete case analysis was used, with missing values less than 10% for included variables. The statistical level of significance of the tests was set to 0.05. The statistical program R, v. 3.6.0 and Python were used to analyze and clean the data.
Results
A total of 1183 PWH with at least two LSM were included, and their baseline characteristics by liver fibrosis progression status are summarized in Table 1. Among these individuals, CAP was available in 1038 cases and MASLD criteria (BMI, diabetes, hypertension, dyslipidemia, insulin resistance) in 778 cases, resulting in a subset of 633 patients where it was possible to assess whether or not they met the MASLD criteria. In this subgroup, 307 (48.5%) fulfilled the MASLD criteria. Supplementary Table S1 compares people with MASLD criteria with people with missing MASLD criteria. In the entire cohort, 25.2% of patients had viral hepatitis coinfection (3.6% with HBV and 21.6% with HCV). Most patients with HCV (78%) were treated and cured at the time of the analysis. In this subset of patients with HCV coinfection, CAP was measured after achieving a sustained viral response. Most patients with HBV (85%) had undetectable HBV DNA. At baseline, the prevalence of significant liver fibrosis and cirrhosis by LSM was 14.4 and 5.7%, respectively, whereas hepatic steatosis was present in 406 out of 1038 patients with available CAP (39.1%). Table 2 shows the differences in baseline characteristics between PWH with and without MASLD.
Table 1.
Baseline characteristics of the whole cohort (n = 1183) by liver fibrosis progression status.
| Total cohort (n = 1183) | Liver fibrosis progression (n = 121) | No liver fibrosis progression (n = 1062) | P | |
| Demographic and anthropometric characteristics | ||||
| Age (years) | 52.9 (46.3–58.2) | 51.7 (44.6–58.8) | 53.0 (46.6–58.2) | 0.77 |
| Male sex (%) | 917 (77.5) | 101 (83.5) | 816 (76.8) | 0.10 |
| Ethnicity (n = 1148, n = 117 and n = 1031, respectively) (%) | ||||
| White | 1021 (88.9) | 102 (87.2) | 919 (89.1) | 0.82 |
| Black | 76 (6.6) | 9 (7.7) | 67 (6.5) | |
| Other | 51 (4.4) | 6 (5.1) | 45 (4.4) | |
| BMI (n = 1081, n = 105 and n = 976, respectively) (kg/m2) | 24.2 (22–26.5) | 26.0 (23.2–29.0) | 24.1 (21.9–26.2) | <0.001 |
| BMI categories (n = 1081, n = 105 and n = 976, respectively) (%) | ||||
| Under/normal weight | 644 (59.6) | 39 (37.1) | 605 (62.0) | <0.001 |
| Overweight | 351 (32.5) | 47 (44.8) | 304 (31.1) | |
| Obesity | 86 (8.0) | 19 (18.1) | 67 (6.9) | |
| HIV-related variables | ||||
| CD4 (cell/μl) | 623.5 (450.2–817.5) | 601 (384–798) | 626 (457–818) | 0.45 |
| Nadir CD4+ (cell/μl) | 200 (96–311) | 176 (95–305) | 200 (96–311) | 0.65 |
| Time since HIV diagnosis (years) | 18.0 (9–26.9) | 15 (8.0–25.2) | 18.4 (9.1–27.0) | 0.04 |
| Undetectable HIV viral load (n = 717, n = 95 and n = 622, respectively) (≤50 copies) (%) | 614 (85.6) | 75 (78.9) | 539 (86.7) | 0.05 |
| Current ART | ||||
| NNRTI (%) | 344 (29.1) | 30 (24.8) | 314 (29.6) | 0.27 |
| NRTI (%) | 910 (76.9) | 92 (76.0) | 818 (77.0) | 0.81 |
| PI (%) | 384 (32.5) | 45 (37.2) | 339 (31.9) | 0.24 |
| INSTI (%) | 528 (44.6) | 48 (39.7) | 480 (45.2) | 0.25 |
| TAF (%) | 191 (16.1) | 16 (13.2) | 175 (16.5) | 0.36 |
| Past exposure to d-drugs (%) | 160 (13.5) | 14 (11.6) | 146 (13.7) | 0.51 |
| Biochemical parameters | ||||
| Platelets (109/l) | 206.0 (171–244) | 203.5 (165.2, 238.8) | 206.0 (172.0, 244.0) | 0.46 |
| Albumin (g/l) | 45.0 (42.9–47) | 43.5 (41.6, 46.7) | 45.0 (43.0, 47.0) | 0.03 |
| ALT (IU/l) | 26.0 (19.0, 37.0) | 36.0 (25.0, 49.0) | 25.0 (18.0, 35.0) | <0.001 |
| AST (IU/l) | 24.0 (19–29) | 27.0 (22.0, 41.0) | 23.0 (19.0, 29.0) | <0.001 |
| Total cholesterol (mmol/l) | 4.7 (4–5.4) | 4.6 (3.8, 5.4) | 4.7 (4.0, 5.4) | 0.41 |
| HDL cholesterol (mmol/l) | 1.2 (1–1.5) | 1.1 (1.0, 1.3) | 1.2 (1.0, 1.5) | <0.001 |
| Triglycerides (mmol/l) | 1.4 (1–2.1) | 1.7 (1.3, 2.8) | 1.3 (0.9, 2.0) | <0.001 |
| Comorbidities | ||||
| Hypertension (n = 808, n = 102 and n = 706, respectively) (%) | 407 (50.4) | 45 (44.1) | 362 (51.3) | 0.17 |
| Type 2 diabetes (n = 619, n = 90 and n = 530, respectively) (%) | 169 (27.3) | 21 (23.3) | 148 (27.9) | 0.37 |
| Cardiovascular disease (n = 529, n = 85 and n = 445, respectively) (%) | 63 (11.9) | 14 (16.5) | 49 (11.0) | 0.15 |
| Liver-related variables | ||||
| HBV coinfection (%) | 42 (3.6) | 10 (8.3) | 32 (3.0) | 0.003 |
| HCV coinfection (%) | 255 (21.6) | 32 (24.8) | 225 (21.2) | 0.36 |
| MASLD (n = 633, n = 70 and n = 562, respectively) (%) | 307 (48.5) | 46 (65.7) | 261 (46.4) | 0.002 |
| LSM (kPa) | 5.3 (4.3–6.5) | 6.5 (5.3, 8.6) | 5.1 (4.2, 6.3) | 0.13 |
| CAP (n = 1038) (dB/m) | 235 (204–274) | 269.0 (226.0, 304.5) | 232.0 (203.0, 268.5) | <0.001 |
| FIB-4 >2.67 (%) | 283 (23.9) | 28 (23) | 255 (24) | 0.84 |
Continuous variables are expressed as median (interquartile range) and categorical variables are expressed as frequencies (%). The P values refer to Mann–Whitney and Kruskal–Wallis tests or χ 2 test between no fibrosis progression and fibrosis progression. ALT, alanine aminotransferase; ART, antiretroviral therapy; AST, aspartate aminotransferase; CAP, controlled attenuation parameter; d-drugs, dideoxynucleoside-drugs; FIB-4, fibrosis 4 index; HBV, hepatitis B virus; HCV, hepatitis C virus; HDL, high-density lipoprotein; INSTI, integrase strand transfer inhibitors; IU, international units; LSM, liver stiffness measurement; MASLD, metabolic dysfunction-associated steatotic liver disease; NNRTI, nonnucleoside reverse transcriptase inhibitors; NRTI, nucleoside reverse transcriptase inhibitors; PI, protease inhibitors; TAF, tenofovir alafenamide.
Table 2.
Baseline characteristics of the cohort by metabolic dysfunction-associated steatotic liver disease status (n = 633).
| MASLD (n = 307) | No MASLD (n = 326) | P | |
| Demographic and anthropometric characteristics | |||
| Age (years) | 55.1 (50.0–59.9) | 46.0 (38.3–53.8) | <0.001 |
| Male sex (%) | 261 (85.0) | 251 (77.0) | 0.01 |
| BMI (kg/m2) | 26.8 (25.3–29.1) | 24.1 (22.1–25.8) | <0.001 |
| BMI categories (%) | |||
| Under/normal weight | 64 (20.9) | 191 (58.6) | <0.001 |
| Overweight | 187 (60.9) | 128 (39.1) | |
| Obesity | 56 (18.2) | 7 (2.3) | |
| HIV-related variables | |||
| CD4+ (cell/μl) | 662 (509–863.5) | 559 (399.5–741.2) | <0.001 |
| Nadir CD4+ (cell/μl) | 196 (100–296.5) | 226.5 (97.5–334.8) | 0.205 |
| Time since HIV diagnosis (years) | 21.1 (11.4–28.5) | 10.0 (5.0–17.7) | <0.001 |
| Undetectable HIV viral load (≤50copies) (%) | 156 (94.0) | 229 (84.8) | 0.004 |
| HIV transmission (%) | |||
| IVDU | 55 (17.9%) | 33 (10.1%) | 0.048 |
| MSM | 138 (45%) | 176 (54.0%) | |
| Heterosexual | 114 (37.1%) | 117 (35.6%) | |
| Current ART (%) | |||
| NNRTI | 96 (31.3) | 99 (30.4) | 0.806 |
| PI | 82 (26.7) | 116 (35.6) | 0.016 |
| INSTI | 176 (57.3) | 72 (22.1) | <0.001 |
| TAF | 55 (17.9) | 20 (6.1) | <0.001 |
| Past exposure to d-drugs (%) | 57 (18.6) | 11 (3.4) | <0.001 |
| Biochemical parameters | |||
| Platelets (109/l) | 205.5 (174–245.2) | 215.0 (181.5–254) | 0.057 |
| ALT (IU/l) | 28.0 (21.0–39.2) | 29.0 (23.0–38.0) | 0.362 |
| AST (IU/l) | 25.0 (20–30) | 23 (19–28) | 0.818 |
| Comorbidities | |||
| Hypertension (n = 213 and n = 286, respectively) (%) | 154 (72.3) | 53 (18.5) | <0.001 |
| Type 2 diabetes (n = 152 and n = 278, respectively) (%) | 83 (54.6) | 9 (3.2) | <0.001 |
| Cardiovascular disease (n = 103 and n = 273, respectively) (%) | 26 (25.2) | 12 (4.4) | <0.001 |
| Liver-related variables | |||
| HBV coinfection (%) | 4 (1.3) | 22 (6.7) | <0.001 |
| HCV coinfection (%) | 56 (18.2) | 46 (14.1) | 0.158 |
| LSM >8 kPa (%) | 57 (18.6) | 22 (6.7) | <0.001 |
| LSM >13 kPa (%) | 18 (5.9) | 9 (2.8) | <0.001 |
| CAP (dB/m) | 287.0 (267.0–316.0) | 218.0 (189.2–243.0) | <0.001 |
Continuous variables are expressed as median (interquartile range) and categorical variables are expressed as frequencies (%). The P values refer to Mann–Whitney and Kruskal–Wallis tests or χ 2 test between no fibrosis progression and fibrosis progression. ALT, alanine aminotransferase; ART, antiretroviral therapy; AST, aspartate aminotransferase; CAP, controlled attenuation parameter; HBV, hepatitis B virus; HCV, hepatitis C virus; HDL, high-density lipoprotein; INSTI, integrase strand transfer inhibitors; IU, international units; IVDU, intravenous drug use; LSM, liver stiffness measurement; MASLD, metabolic dysfunction-associated steatotic liver disease; NNRTI, nonnucleoside reverse transcriptase inhibitors; NRTI, nucleoside reverse transcriptase inhibitors; PI, protease inhibitors; TAF, tenofovir alafenamide.
Transitions across fibrosis stages
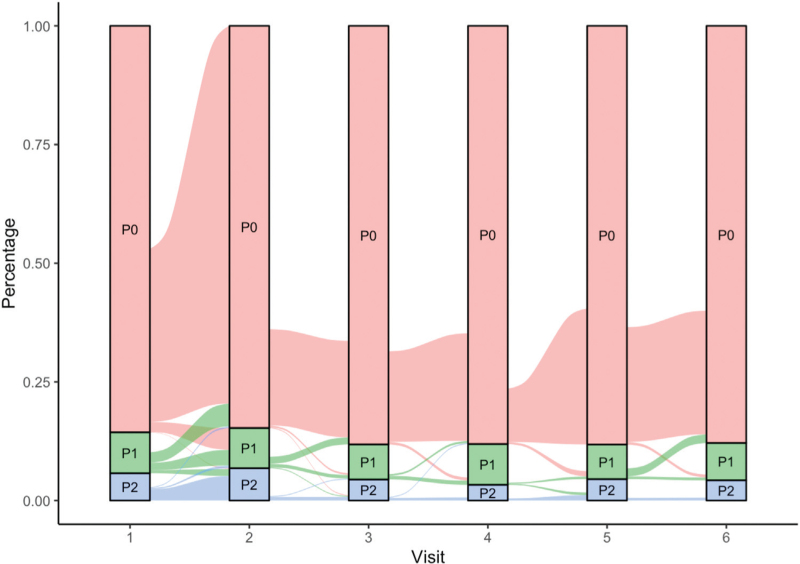
During a median follow-up period of 2.5 (1.9–3.5) years, a minimum of two and a maximum of six yearly transient elastography examination were performed. An alluvial plot was used to visualize the transition of patients across liver fibrosis stages (no fibrosis, significant liver fibrosis, cirrhosis) during the follow-up visits (Fig. 1). Overall, the cumulative incidence of liver fibrosis progression and regression was 8% (95% CI 6.5–9.9) and 5.2% (95% CI 4.3–6.3), respectively. The incidence rate of liver fibrosis progression and regression was 2.8 per 100 person-years (95% CI 2.3–3.4) and 2.2 per 100 person-years (95% CI 1.9–2.6), respectively. The incidence rate of progression to cirrhosis was 0.2 per 100 person-years (95% CI 0.1–0.5). Patients who developed liver fibrosis progression at follow-up had higher BMI, less frequently undetectable HIV viral load, lower albumin, higher ALT and AST, lower HDL cholesterol, higher triglycerides, and higher CAP. They also had higher prevalence of HBV coinfection and MASLD, while there was no difference in LSM and in prevalence of FIB-4 greater than 2.67 (Table 1). The Markov model describing the transition across liver fibrosis stages is reported in Table 3. Overweight at baseline (OR 1.12, 95% CI 1.04–1.21) and weight gain (OR 3.11, 95% CI 1.59–6.08) were positively associated with fibrosis progression. Similarly, weight gain (OR 0.30, 95% CI 0.10–0.84), as well as male sex (OR 0.32, 95% CI 0.14–0.75), reduced the probability of fibrosis regression. HBV and HCV coinfection, HIV-related variables, and type of current ART were not associated with changes in liver fibrosis. Since there may a plausible basis for correlating BMI with LSM error, we have performed the sensitivity analysis which included only PWH who transitioned from LSM less than 8 to LSM greater than 10 kPa. Even in this sensitivity analysis, weight gain was confirmed as a major driver of liver fibrosis progression (data not shown).
Fig. 1.
Alluvial plot showing the transition of patients through the stages of liver fibrosis during follow-up visits.
P0, absence of significant liver fibrosis (liver stiffness measurement <8 kPa); P1, significant liver fibrosis (liver stiffness measurement at least 8 kPa and less than 13 kPa); P2, cirrhosis (liver stiffness measurement ≥13 kPa).
Table 3.
Markov model describing transitions of liver fibrosis (progression or regression) (n = 1183).
| Fibrosis progression (aOR, 95% CI) | Fibrosis regression (aOR, 95% CI) | |
| Age >50 years (yes vs. no) | 0.99 (0.95–1.03) | 0.99 (0.95–1.02) |
| Males (yes vs. no) | 0.87 (0.36–2.09) | 0.32 (0.14–0.75) |
| Overweight (yes vs. no) | 1.12 (1.04–1.21) | 1.00 (0.90–1.11) |
| Weight gain (yes vs. no) | 3.11 (1.59–6.08) | 0.30 (0.10–0.84) |
| Years since HIV diagnosis >10 years (yes vs. no) | 1.09 (0.40–2.95) | 1.19 (0.43–3.33) |
| Nadir CD4+ cell count <200 cell/μl (yes vs. no) | 1.03 (0.53–2.03) | 0.78 (0.35–1.74) |
| HBV coinfection (yes vs. no) | 1.79 (0.52–6.20) | 0.30 (0.04–2.51) |
| HCV coinfection (yes vs. no) | 1.65 (0.79–3.44) | 0.63 (0.29–1.39) |
| Current exposure to INSTI (yes vs. no) | 0.61 (0.26–1.45) | 0.73 (0.34–1.58) |
| Current exposure to protease inhibitors (yes vs. no) | 0.85 (0.35–2.06) | 1.17 (0.55–2.50) |
| Current exposure to NNRTI (yes vs. no) | 0.41 (0.15–1.11) | 0.99 (0.45–2.18) |
| Current exposure to TAF (yes vs. no) | 1.11 (0.55–2.26) | 0.96 (0.43–2.14) |
Odds ratios (OR) and 95% confidence intervals (CI) are shown for each variable analyzed.
aOR, adjusted odds ration; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; INSTI, integrase strand transfer inhibitors; NNRTI, nonnucleoside reverse transcriptase inhibitors; TAF, tenofovir alafenamide.
Predictors of fibrosis progression by multivariable analysis
Table 4 reports the Cox proportional hazard model of predictors of liver fibrosis progression. After adjustments, MASLD (adjusted hazard ratio 2.72, 95% CI 1.05–7.02) and weight gain (adjusted hazard ratio 3.12, 95% CI 1.41–6.90) were independent predictors of fibrosis progression. Conversely, consistent with the Markov model, treated coinfections with HBV or HCV, as well as HIV-related variables and current ART regimen, did not predict liver fibrosis progression.
Table 4.
Multivariable Cox regression analysis of predictors of liver fibrosis progression (n = 633).
| Predictors | aHR (95% CI) | P |
| Male sex (yes vs. no) | 1.10 (0.55–4.13) | 0.424 |
| Age (per year) | 0.98 (0.93–1.04) | 0.430 |
| Nadir CD4+ cell count <200 cell/μl (yes vs. no) | 0.56 (0.24–1.29) | 0.172 |
| Time since HIV diagnosis (per year) | 1.05 (1.00–1.11) | 0.059 |
| HBV coinfection (yes vs. no) | 3.26 (0.62–17.04) | 0.162 |
| HCV coinfection (yes vs. no) | 0.89 (0.31–2.56) | 0.826 |
| MASLD (yes vs. no) | 2.72 (1.05–7.02) | 0.039 |
| Weight gain (yes vs. no) | 3.12 (1.41–6.90) | 0.005 |
| Current exposure to INSTI (yes vs. no) | 1.51 (0.55–4.13) | 0.424 |
| Current exposure to TAF (yes vs. no) | 0.66 (0.26–1.65) | 0.371 |
| Current exposure to NNRTI (yes vs. no) | 0.58 (0.19–1.81) | 0.348 |
| Current exposure to PI (yes vs. no) | 1.37 (0.51–3.65) | 0.531 |
aHR, adjusted hazard ratio; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; INSTI, integrase strand transfer inhibitors; MASLD, metabolic dysfunction-associated steatotic liver disease; NNRTI, nonnucleoside reverse transcriptase inhibitors; PI, protease inhibitors; TAF, tenofovir alafenamide.
Discussion
In this multicentric longitudinal cohort study, we found that liver fibrosis progression is frequent among PWH. MASLD was diagnosed in 48.5% of PWH with available CAP and cardiometabolic data, and it was a significant predictor of liver fibrosis progression, together with other metabolic health variables, namely overweight and weight gain. In addition, weight gain and male sex prevented the regression of liver fibrosis, while treated viral hepatitis coinfection and current ART regimens were not associated with transitions in liver fibrosis. Our findings support the current guidelines of the European AIDS Clinical Society, which recommend that PWH with metabolic abnormalities should be screened for liver fibrosis regardless of viral hepatitis coinfection [7]. Notably, PWH are still not listed as a high-risk group for hepatic steatosis in international MASLD guidelines [12,19], and they are currently excluded from therapeutic trials for MASH [20], being often left behind in the global effort for liver fibrosis screening and management [21]. We advocate for multidisciplinary care of HIV patients, with an increased involvement of hepatology providers, to halt and manage liver fibrosis in PWH.
Our study focused on liver fibrosis, which is the hallmark event in the natural history of any chronic liver disease, leading to cirrhosis, liver failure and hepatocellular carcinoma [3]. Liver fibrosis plays a critical role in patient risk stratification, as it is known to predict hepatic and extrahepatic complications as well as all-cause mortality [22]. Given the dynamic nature of liver fibrosis, in the present study, rather than a single snapshot, we provided a continuum of its transitions throughout the patients’ follow-up. Our findings show that liver fibrosis progression is common in PWH, with an incidence rate of 2.8 per 100 person-years (95% CI, 2.3–3.4). Conversely, the rate of fibrosis regression was 2.2 per 100 person-years (95% CI 1.9–2.6), lower than in HIV-uninfected patients with MASLD [23]. These results reflect the high level of complexity underlying the multifaceted pathogenesis of liver fibrosis in PWH, including a higher prevalence of classic metabolic risk factors and HIV-unique factors such as virus-related inflammation and exposure to ART [6]. Regarding the latter, our results confirm that past exposure to d-drugs is more frequent in PWH with vs. without MASLD (18.4 vs. 3.4%, P < 0.001), with a possible role of these drugs in lipodystrophy-induced metabolic alterations that may persist even after discontinuation [24]. Although HCV coinfection remains the leading cause of liver-related mortality in PWH, this paradigm is rapidly changing, with a decline in HCV-related deaths because of the new curative option of direct-acting antivirals and an increasing burden of MASLD that mirrors the epidemic of metabolic syndrome [2]. Thus, a critical goal in HIV research is to clarify the role of metabolic comorbidities in the dynamics of liver fibrosis, with a special focus on the impact of weight gain, which has been observed in PWH treated with new ART regimens [8–10], and MASLD. Our results contribute significantly to this debate by showing that overweight, weight gain, and MASLD, but not viral hepatitis coinfection, are predictors of liver fibrosis progression in PWH, with weight gain also preventing fibrosis regression. This possibly reflects the successful treatment of HCV and the adequate management of chronic HBV infection in our cohort, mainly with tenofovir disoproxil fumarate (TDF) and TAF.
Weight change is critical to the development and management of liver fibrosis. Indeed, lifestyle intervention is the first-line therapy for MASLD in both the general population and PWH [12,25], and weight loss is known to reverse liver fibrosis [26]. Weight gain in PWH has a complex and not fully understood pathogenesis. Initiation of ART is often followed by weight gain, especially in patients with late HIV presentation at baseline [27,28], which can be interpreted as a ‘return to health’ phenomenon, as the reduction of systemic inflammation decreases metabolic demand [29]. In addition, the improved tolerability of new ART regimens may increase patients’ appetite, leading to weight gain [30]. However, there may also be a cellular mechanism underlying the association between ART and gaining weight. For instance, INSTI were found to have an inhibitory effect on α-melanocyte-stimulating hormone, which can interfere with food regulation and cause obesity, but only at higher doses than those used in clinical practice [31]. Interestingly, INSTI were shown to play a role in adipogenesis, lipogenesis, oxidative stress, and insulin resistance, all possible causes of weight gain, although further in-vivo studies are warranted [32]. Finally, host-related factors are also relevant to weight gain in PWH, with older age and high-income country of origin being positively associated with gaining weight, and still conflicting findings on the role of sex differences and BMI at ART initiation [30]. In our study, although weight gain increased the risk of liver fibrosis progression and prevented its regression, we did not find an association between any ART regimen and liver fibrosis changes. In fact, although INSTI and TAF can cause weight gain, their impact on clinical outcomes is still controversial. Although Neesgaard et al. [33] observed an association between INSTI and an excess incidence of cardiovascular disease, this association was not confirmed by Surial et al. [34]. Oppositely, Milic et al. [35] showed a protective effect of INSTI on insulin resistance in PWH without metabolic abnormalities, aligned with O’Halloran et al. who documented a lower risk for cardiovascular events among 20 000 treatment-naive PWH who started INSTI-based ART compared with those who started other ART classes [36]. Similarly, evidence regarding the role of INSTI on MASLD is conflicting, with two studies conducted in HIV/HCV-coinfected patients showing reduced hepatic steatosis after INSTI switch [37,38], one suggesting INSTI and TAF as independent predictors of incident hepatic steatosis [39], and another reporting no changes in liver fat [10]. Therefore, in this heated debate about the metabolic and hepatic effects of the new ART regimes, our findings did not suggest an association between current ART exposure and liver fibrosis progression. However, prior or cumulative exposure to ART were not assessed, thus future larger studies addressing these issues and focusing on liver-related outcomes are warranted to better understand the relationship between ART and MASLD with or without liver fibrosis.
To our knowledge, this is the first study to investigate the recently proposed definition of MASLD in PWH. Interestingly, MASLD may better characterize hepatic steatosis than NAFLD in PWH [11]. First, as a positive definition, MASLD allows for the coexistence of other liver diseases, such as viral hepatitis coinfection, which is common in PWH and accounted for a quarter of our cohort. Second, MASLD better highlights the pathophysiological relationship between hepatic steatosis and metabolic comorbidities, such as hypertension and diabetes, which are frequent in PWH, with a prevalence of 50.4 and 27.3% in our study population. Finally, we previously reported a high prevalence of lean MASLD in PWH [40], and the new definition of MASLD may provide a better risk stratification of lean individuals, with a meaningful distinction between those who are metabolically healthy and metabolically unhealthy [11].
Finally, our results show that men had lower rates of liver fibrosis regression compared with women. Indeed, liver fibrosis is known to be a sex-dimorphic disease, as its prevalence is higher in men and women after menopause, suggesting a protective effect of estrogens [41]. In line with our findings, a cross-sectional study of 544 African American patients showed that men with HIV had increased likelihood of exhibiting liver fibrosis compared with women [42].
Our study has several strengths. First, the longitudinal cohort design with multiple transient elastography examinations allowed to capture the dynamics of liver fibrosis. Second, the large and diverse population increased the generalizability of our findings. Several limitations of our study should be considered. First, liver fibrosis was assessed by transient elastography, as the gold standard of liver biopsy is not feasible on a large-scale basis. Second, there was a significant amount of missing data that prevented a definitive diagnosis of MASLD during follow-up visits. Third, the relatively short follow-up, with a lack of assessment of hepatic and extrahepatic outcomes, did not allow us to explore the role of liver fibrosis in predicting clinical outcomes. Fourth, we assessed only current exposures to ART classes and not previous or cumulative exposures, which may have a different impact on liver fibrosis progression and regression. For instance, we could not account for the effect of INSTI on weight gain, which may be more pronounced during the first year and in women, especially of Black ethnicity [41,42]. Fifth, as very few PWH had current exposure to TDF, we were not able to assess the interaction among weight gain/loss, TDF and liver fibrosis changes. Sixth, we had a male predominance and lack of information on menopause in our cohort. Seventh, the prevalence of MASLD is likely overestimated, as MASLD was calculated only in PWH with available both CAP and cardiometabolic data, therefore, selection bias cannot be excluded. Eighth, there may be an overestimation of the prevalence of significant liver fibrosis in patients with vs. without MASLD, as the severity of steatosis has been shown to influence LSM in patients with MASLD [43]. Finally, the lack of more detailed information on alcohol intake is a limitation; however, we excluded patients with significant alcohol intake because we were interested in assessing the specific role of HIV-associated MASLD rather than alcohol-induced steatotic liver.
In conclusion, liver fibrosis progression is common in PWH, especially in those with metabolic conditions. MASLD accelerates liver fibrosis progression along with weight gain, which also prevents its regression. In this at-risk population, liver fibrosis should be promptly recognized and monitored beyond viral hepatitis coinfection.
Acknowledgements
G.G. and J.M. contributed to conception, study design, data and interpretation of the data. S.R. and F.M. contributed to interpretation of the data and statistical analysis. J.B., A.D., J.C., F.M., M.D.M., D.K., W.E., S.C., C.M., J.R. contributed to data and interpretation of data. F.C. contributed to interpretation of the data and first draft of the manuscript. G.S. contributed to conception, study design, data and interpretation of the data and first draft of the manuscript. All authors approved the final version of the article. Part of this work has been presented at the Conference on Retroviruses and Opportunistic Infections (CROI) (Seattle, Washington, USA; February 2023).
Financial support: the study was not supported. J.B. is supported by a scholarship of the BONFOR research support program for young scientists at the Rheinische Friedrich-Wilhelms-Universitat (BONFOR Funding Instrument 1, Type A; Application number: 2020 1A-08). W.E. has received MSc fellowship from the Canadian Network on Hepatitis C (CanHepC). CanHepC is funded by a joint initiative of the Canadian Institutes of Health Research (NPC-178912) and the Public Health Agency of Canada. Giada Sebastiani is supported by a Senior Salary Award from Fonds de Recherche du Quebec – Sante (FRQS) (#296306).
Conflicts of interest
G.G. received a research grant and speaker honoraria from Gilead, ViiV, Merck and Jansen and attended advisory boards of Gilead, ViiV and Merck. J.R. has received honoraria for consulting or speaking at educational events from Abvie, Boehringer, Galapagos, Gilead, Merck, Janssen, Theratechnologies and ViiV. G.S. has acted as speaker for Merck, Gilead, Abbvie, Novo Nordisk, Pfizer, served as an advisory board member for Pfizer, Merck, Novo Nordisk, Gilead, and has received unrestricted research funding from Theratecnologies Inc. J.M., S.R., F.M., F.C., J.B., A.D., J.C., F.M., M.D.M., D.K., W.E., S.C., C.M. have nothing to disclose.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
References
- 1. Croxford S, Kitching A, Desai S, Kall M, Edelstein M, Skingsley A, et al. Mortality and causes of death in people diagnosed with HIV in the era of highly active antiretroviral therapy compared with the general population: an analysis of a national observational cohort . Lancet Public Health 2017; 2:e35–e46. [DOI] [PubMed] [Google Scholar]
- 2. Paik JM, Henry L, Golabi P, Alqahtani SA, Trimble G, Younossi ZM. Presumed nonalcoholic fatty liver disease among Medicare beneficiaries with HIV, 2006–2016 . Open Forum Infect Dis 2020; 7:ofz509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taylor RS, Taylor RJ, Bayliss S, Hagström H, Nasr P, Schattenberg JM, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis . Gastroenterology 2020; 158:1611.e12–1625.e12. [DOI] [PubMed] [Google Scholar]
- 4. Kalligeros M, Vassilopoulos A, Shehadeh F, Vassilopoulos S, Lazaridou I, Mylonakis E, et al. Prevalence and characteristics of nonalcoholic fatty liver disease and fibrosis in people living with HIV monoinfection: a systematic review and meta-analysis . Clin Gastroenterol Hepatol 2023; S1542-3565:1708–1722. [DOI] [PubMed] [Google Scholar]
- 5. Kaspar MB, Sterling RK. Mechanisms of liver disease in patients infected with HIV . BMJ Open Gastroenterol 2017; 4:e000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cervo A, Shengir M, Patel K, Sebastiani G. NASH in HIV . Curr HIV/AIDS Rep 2020; 17:601–614. [DOI] [PubMed] [Google Scholar]
- 7. Ambrosioni J, Levi L, Alagaratnam J, Van Bremen K, Mastrangelo A, Waalewijn H, et al. EACS Governing Board. Major revision version 12.0 of the European AIDS Clinical Society guidelines 2023 . HIV Med 2023; 24:1126–1136. [DOI] [PubMed] [Google Scholar]
- 8. Bourgi K, Jenkins CA, Rebeiro PF, Palella F, Moore RD, Altoff KN, et al. Weight gain among treatment-naïve persons with HIV starting integrase inhibitors compared to nonnucleoside reverse transcriptase inhibitors or protease inhibitors in a large observational cohort in the United States and Canada . J Int AIDS Soc 2020; 23:e25484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sax PE, Erlandson KM, Lake JE, Mccomsey GA, Orkin C, Esser S, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials . Clin Infect Dis 2020; 71:1379–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hanttu A, Vuoti S, Kivelä P, Lundbom N, Hakkarainen A, Lundbom J, et al. Liver fat, adipose tissue, and body composition changes after switching from a protease inhibitor or efavirenz to raltegravir . AIDS Patient Care STDS 2021; 35:335–341. [DOI] [PubMed] [Google Scholar]
- 11. Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal A, Kanwal F, et al. NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature . Hepatology 2023; 79:1542–1556. [DOI] [PubMed] [Google Scholar]
- 12. Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, et al. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease . Hepatology 2023; 77:1797–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pembroke T, Deschenes M, Lebouché B, Benmassaoud A, Sewitch M, Ghali P, et al. Hepatic steatosis progresses faster in HIV mono-infected than HIV/HCV co-infected patients and is associated with liver fibrosis . J Hepatol 2017; 67:801–808. [DOI] [PubMed] [Google Scholar]
- 14. Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Lédinghen V, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis . J Hepatol 2017; 66:1022–1030. [DOI] [PubMed] [Google Scholar]
- 15. European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Clinical Practice Guidelines on noninvasive tests for evaluation of liver disease severity and prognosis – 2021 update . J Hepatol 2021; 75:659–689.34166721 [Google Scholar]
- 16. Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C . Gastroenterology 2012; 142:1293.e4–1302.e4. [DOI] [PubMed] [Google Scholar]
- 17. Benmassaoud A, Nitulescu R, Pembroke T, Halme AS, Ghali P, Deschenes M, et al. Liver-related events in human immunodeficiency virus–infected persons with occult cirrhosis . Clin Infect Dis 2019; 69:1422–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perazzo H, Cardoso SW, Yanavich C, Nunes EP, Morata M, Gorni N, et al. Predictive factors associated with liver fibrosis and steatosis by transient elastography in patients with HIV mono-infection under long-term combined antiretroviral therapy . J Int AIDS Soc 2018; 21:e25201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. EASL–EASD–EASO Clinical Practice Guidelines for the management of nonalcoholic fatty liver disease . J Hepatol 2016; 64:1388–1402. [DOI] [PubMed] [Google Scholar]
- 20. Guaraldi G, Maurice JB, Marzolini C, Monteith K, Milic J, Tsochatzis E, et al. SHIVER Network. New drugs for NASH and HIV infection: great expectations for a great need . Hepatology 2020; 71:1831–1844. [DOI] [PubMed] [Google Scholar]
- 21. Sebastiani G, Milic J, Tsochatzis EA, Marzolini C, Betel M, Bhagani S, et al. Steatohepatitis in HIV Emerging Research (SHIVER) Network. Letter to the editor: people living with HIV and NAFLD: a population left behind in the global effort for liver fibrosis screening? . Hepatology 2023; 78:E87–E88. [DOI] [PubMed] [Google Scholar]
- 22. Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic Review and Meta-analysis . Hepatology 2017; 65:1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Le P, Payne JY, Zhang L, Deshpande A, Rothberg MB, Alkhouri N, et al. Disease state transition probabilities across the spectrum of NAFLD: a systematic review and meta-analysis of paired biopsy or imaging studies . Clin Gastroenterol Hepatol 2023; 21:1154–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rockstroh JK, Mohr R, Behrens G, Spengler U. Liver fibrosis in HIV: which role does HIV itself, long-term drug toxicities and metabolic changes play? . Curr Opin HIV AIDS 2014; 9:365–370. [DOI] [PubMed] [Google Scholar]
- 25. Cinque F, Cespiati A, Lombardi R, Guaraldi G, Sebastiani G. Nutritional and lifestyle therapy for NAFLD in people with HIV . Nutrients 2023; 15:1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis . Gastroenterology 2015; 149:367.e5–378.e5. [DOI] [PubMed] [Google Scholar]
- 27. Barceló C, Guidi M, Thorball CW, Hammer C, Chaouch A, Scherrer AU, et al. Swiss HIV Cohort Study. Impact of genetic and nongenetic factors on body mass index and waist-hip ratio change in HIV-infected individuals initiating antiretroviral therapy . Open Forum Infect Dis 2020; 7:ofz464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tate T, Willig AL, Willig JH, Raper JL, Moneyham L, Kempf MC, et al. HIV infection and obesity: where did all the wasting go? . Antivir Ther 2012; 17:1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taramasso L, Ricci E, Menzaghi B, Orofino G, Passerini S, Madeddu G, et al. Weight gain: a possible side effect of all antiretrovirals . Open Forum Infect Dis 2017; 4:ofx239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guaraldi G, Bonfanti P, Di Biagio A, Gori A, Mili? J, Saltini P, et al. Evidence gaps on weight gain in people living with HIV: a scoping review to define a research agenda . BMC Infect Dis 2023; 23:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McMahon C, Trevaskis JL, Carter C, Holsapple K, White K, Das M, et al. Lack of an association between clinical INSTI-related body weight gain and direct interference with MC4 receptor (MC4R), a key central regulator of body weight . PLoS One 2020; 15:e0229617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gorwood J, Bourgeois C, Pourcher V, Pourcher G, Charlotte F, Mantecon M, et al. The integrase inhibitors dolutegravir and raltegravir exert proadipogenic and profibrotic effects and induce insulin resistance in human/simian adipose tissue and human adipocytes . Clin Infect Dis 2020; 71:e549–e560. [DOI] [PubMed] [Google Scholar]
- 33. Neesgaard B, Greenberg L, Miró JM, Grabmeier-Pfistershammer K, Wandeler G, Smith C, et al. Associations between integrase strand-transfer inhibitors and cardiovascular disease in people living with HIV: a multicentre prospective study from the RESPOND cohort consortium . Lancet HIV 2022; 9:e474–e485. [DOI] [PubMed] [Google Scholar]
- 34. Surial B, Chammartin F, Damas J, Calmy A, Haerry D, Stöckle M, et al. Impact of integrase inhibitors on cardiovascular disease T events in people with HIV starting antiretroviral therapy . Clin Infect Dis 2023; 77:729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Milic J, Renzetti S, Ferrari D, Barbieri S, Menozzi M, Carli F, et al. Relationship between weight gain and insulin resistance in people living with HIV switching to integrase strand transfer inhibitors-based regimens . AIDS 2022; 36:1643–1653. [DOI] [PubMed] [Google Scholar]
- 36. Ja O, J.S., Am B, Ma O, Wg P. Brief report: integrase strand transfer inhibitors are associated with lower risk of incident cardiovascular disease in people living with HIV . J Acquired Immune Defic Syndr 2020; 84:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Calza L, Colangeli V, Borderi M, Coladonato S, Tazza B, Fornaro G, et al. Improvement in liver steatosis after the switch from a ritonavir-boosted protease inhibitor to raltegravir in HIV-infected patients with nonalcoholic fatty liver disease . Infect Dis (Lond) 2019; 51:593–601. [DOI] [PubMed] [Google Scholar]
- 38. Macías J, Mancebo M, Merino D, Téllez F, Montes-Ramírez ML, Pulido F, et al. Spanish AIDS Research Network-HEP09 Study Group. Changes in liver steatosis after switching from efavirenz to raltegravir among human immunodeficiency virus–infected patients with nonalcoholic fatty liver disease . Clin Infect Dis 2017; 65:1012–1019. [DOI] [PubMed] [Google Scholar]
- 39. Bischoff J, Gu W, Schwarze-Zander C, Boesecke C, Wasmuth JC, van Bremen K, et al. Stratifying the risk of NAFLD in patients with HIV under combination antiretroviral therapy (cART) . EClinicalMedicine 2021; 40:101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cervo A, Milic J, Mazzola G, Schepis F, Petta S, Krahn T, et al. Prevalence, predictors, and severity of lean nonalcoholic fatty liver disease in patients living with human immunodeficiency virus . Clin Infect Dis 2020; 71:e694–e701. [DOI] [PubMed] [Google Scholar]
- 41. Burra P, Bizzaro D, Gonta A, Shalaby S, Gambato M, Morelli MC, et al. Clinical impact of sexual dimorphism in nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) . Liver Int 2021; 41:1713–1733. [DOI] [PubMed] [Google Scholar]
- 42. Zarini G, Sales Martinez S, Campa A, Sherman K, Tamargo J, Hernandez Boyer J, et al. Sex differences, cocaine use, and liver fibrosis among African Americans in the Miami adult studies on HIV Cohort . J Womens Health (Larchmt) 2020; 29:1176–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Petta S, Maida M, Macaluso FS, Di Marco V, Cammà C, Cabibi D, Craxì A. The severity of steatosis influences liver stiffness measurement in patients with nonalcoholic fatty liver disease . Hepatology 2015; 62:1101–1110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.