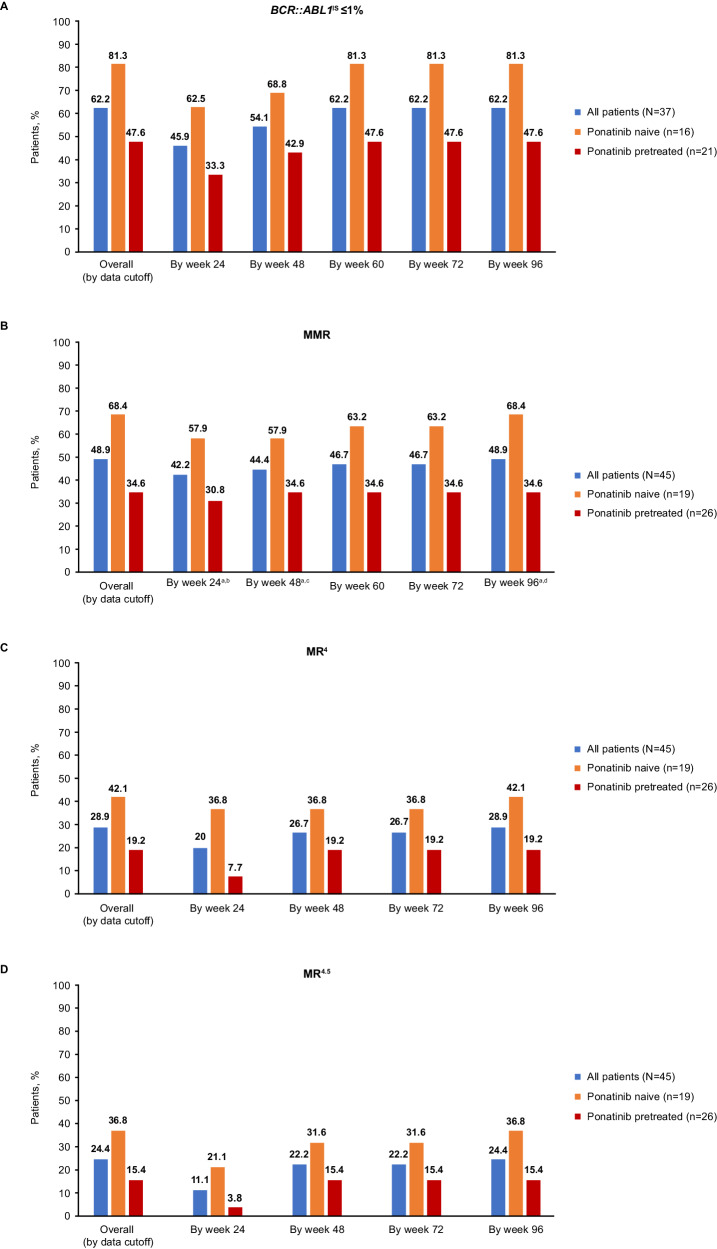
Fig. 1. Cumulative molecular response in ponatinib-pretreated and -naive patients without the indicated response at baseline.
Cumulative (A) BCR::ABL1IS ≤1%, (B) MMR, (C) MR4, and (D) MR4.5 in evaluable patients. IS International Scale, MMR major molecular response, MR4 BCR::ABL1IS ≤0.01%, MR4.5 BCR::ABL1IS ≤0.0032%. a CIs are based on the Clopper–Pearson method. 95% CIs were used for all patients, and 90% CIs were used for ponatinib-pretreated and -naive patients. b The rate of MMR by week 24 was 42.2% (95% CI, 27.7–57.8%) in all patients, 57.9% (90% CI, 36.8–77.0%) in ponatinib-naive patients, and 30.8% (90% CI, 16.3–48.7%) in ponatinib-pretreated patients. c The rate of MMR by week 48 was 44.4% (95% CI, 29.6–60.0%) in all patients, 57.9% (90% CI, 36.8–77.0%) in ponatinib-naive patients, and 34.6% (90% CI, 19.4–52.6%) in ponatinib-pretreated patients. d The rate of MMR by week 96 was 48.9% (95% CI, 33.7–64.2%) in all patients, 68.4% (90% CI, 47.0–85.3%) ponatinib-naive patients, and 34.6% (90% CI, 19.4–52.6%) in ponatinib-pretreated patients.