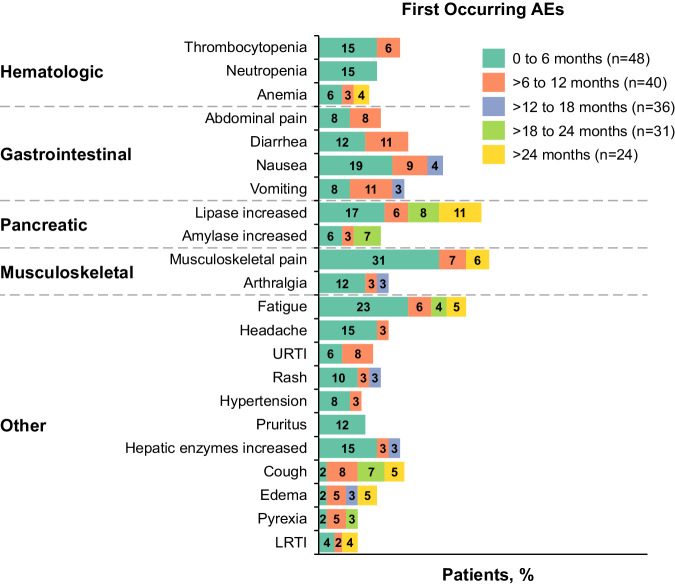
Fig. 4. Adverse reactions (≥10% at first occurrence) over time.
a,bADR adverse drug reaction, AE adverse event, LRTI lower respiratory tract infection, URTI upper respiratory tract infection. a Includes reported AEs and adverse drug reactions. b Proportions are calculated based on the number of patients at risk of an event (ie, patients ongoing treatment and event-free at the start of the interval). A patient with multiple occurrences of an event in the same time interval is counted only once in that time interval. The safety topics correspond to either single preferred terms or groups of preferred terms according to the adverse drug reaction definitions.