Figure 4.
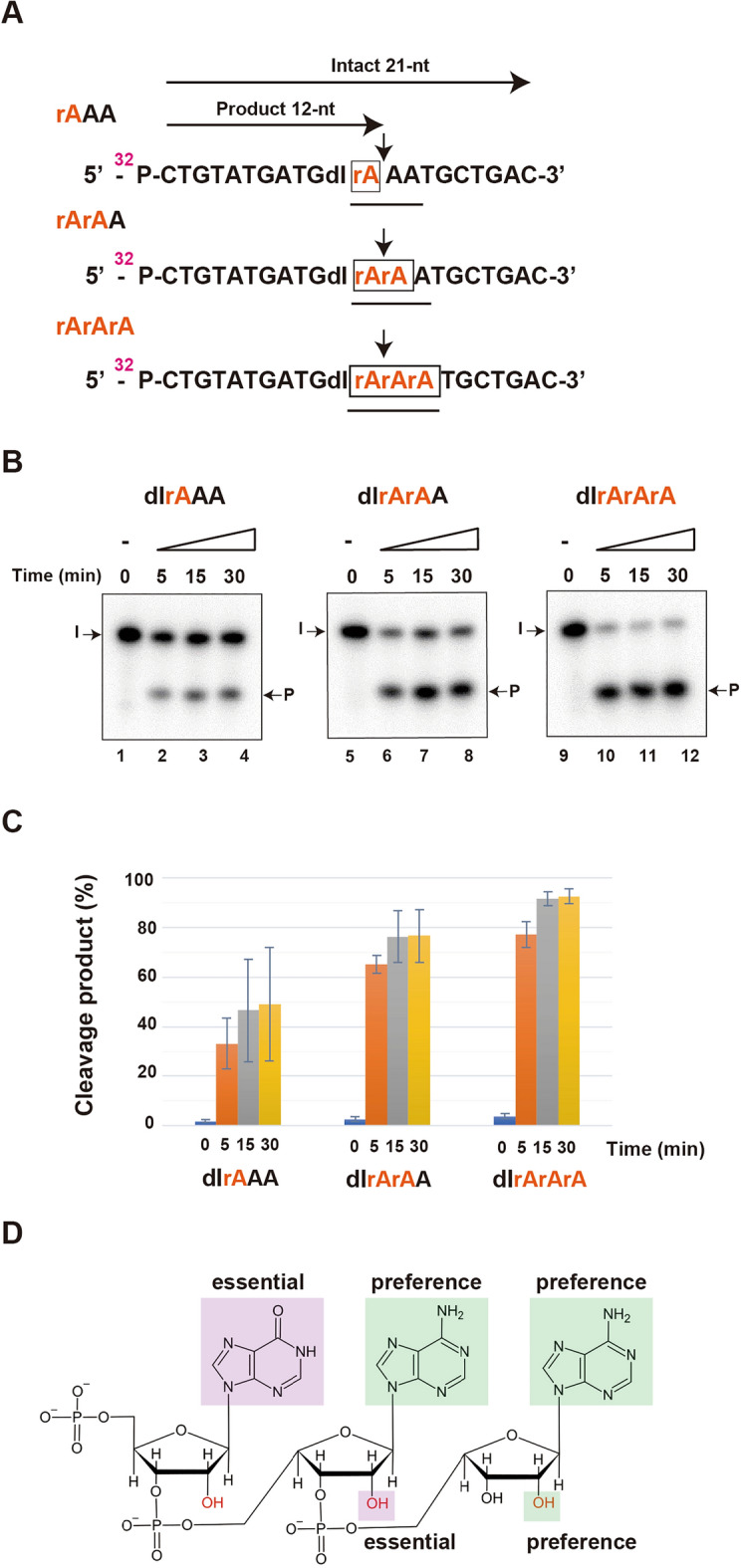
hEndoV prefers a hydroxy (–OH) modification of adenosine. (A) 32P-labeled substrates containing deoxyinosine and a series of adenosine modifications are shown. Intact 21-nt; Intact oligonucleotide. Product 12-nt; cleavage products. (B) 32P-labeled substrate containing deoxyinosine and rAAA (lanes 1–4), rArAA (lanes 5–8) and rArArA (lanes 9–12) were incubated with 5 nM hEndoV at 37 ℃ for 0, 5, 15 and 30 min. (C) The cleavage products were analyzed by denaturing 12.5% urea gel electrophorese. (D) Preference of chemical structure in hEndoV cleavage activity. Abbreviations: hEndoV, human endonuclease V; P, phosphorus; rA, ribo-adenine; dI, deoxyinosine; A, deoxy-adenine Representatives of three independent experiments are shown.
