Abstract
Background
Anthracycline combined with cytarabine has been the standard for induction therapy of newly diagnosed acute myeloid leukaemia (AML) for several decades. Due to theoretical advantages, idarubicin (IDA) might be the most effective and tolerable anthracycline. However, there is no evidence that would definitively prove the superiority of IDA over other anthracyclines.
Objectives
To assess the efficacy and safety of IDA versus other anthracyclines in induction therapy of newly diagnosed AML.
Search methods
We identified relevant randomised controlled trials (RCTs) by searching the Cochrane Central Register of Controlled Trials (The Cochrane Library 2014, Issue 8), MEDLINE (from 1946 to 3 August 2014), EMBASE (from 1974 to 3 August 2014), Chinese BioMedical Literature Database (1978 to 3 August 2014), relevant conference proceedings and databases of ongoing trials.
Selection criteria
RCTs that compared IDA with other anthracyclines in induction therapy of newly diagnosed AML.
Data collection and analysis
Two review authors independently extracted data and assessed the quality of studies according to methodological standards of the Cochrane Collaboration. We estimated hazard ratios (HRs) for time‐to‐event data outcomes using the inverse variance method, and risk ratios (RRs) for dichotomous data outcomes using the Mantel‐Haenszel method. We adopted a fixed‐effect model and repeated the main meta‐analysis by a random‐effects model in a sensitivity analysis.
Main results
We identified 2017 references. Ultimately, 27 RCTs (including 22 two‐armed RCTs and five three‐armed RCTs) involving 9549 patients were eligible. The consolidation treatments adopted in the studies were comparable and had no impact on the results. Overall, the risk of bias of the studies was unclear to high.
Eighteen RCTs (N = 6755) assessed IDA versus daunorubicin (DNR). The main meta‐analyses showed that IDA compared with DNR prolonged overall survival (OS) (12 studies, 5976 patients; HR 0.90, 95% confidence interval (CI) 0.84 to 0.96, P = 0.0008; high quality of evidence) and disease‐free survival (DFS) (eight studies, 3070 patients; HR 0.88, 95% CI 0.81 to 0.96, P = 0.004; moderate quality of evidence), increased complete remission (CR) rate (18 studies, 6692 patients; RR 1.04, 95% CI 1.01 to 1.07, P = 0.009; moderate quality of evidence), and reduced relapse rate (four studies, 1091 patients; RR 0.88, 95% CI 0.80 to 0.98, P = 0.02; moderate quality of evidence), although increased the risks of death on induction therapy (14 studies, 6349 patients; RR 1.18, 95% CI 1.01 to 1.36, P = 0.03; moderate quality of evidence) and grade 3/4 mucositis (five studies, 2000 patients; RR 1.22, 95% CI 1.04 to 1.44, P = 0.02; moderate quality of evidence). There was no evidence for difference in the risks of grade 3/4 cardiac toxicity (six studies, 2795 patients; RR 0.98, 95% CI 0.70 to 1.37, P = 0.91; moderate quality of evidence) and other grade 3/4 adverse events (AEs). None of the studies reported on quality of life (QoL).
Eight RCTs (N = 2419) evaluated IDA versus mitoxantrone (MIT). The main meta‐analyses showed that there was no evidence for difference between arms in OS (six studies, 2171 patients; HR 0.98, 95% CI 0.89 to 1.08, P = 0.69; high quality of evidence), DFS (four studies, 249 patients; HR 0.88, 95% CI 0.70 to 1.10, P = 0.26; low quality of evidence), CR rate (eight studies, 2411 patients; RR 0.97, 95% CI 0.92 to 1.03, P = 0.32;moderate quality of evidence), the risks of death on induction therapy (five studies, 2055 patients; RR 1.10, 95% CI 0.88 to 1.38, P = 0.39; moderate quality of evidence) and relapse (three studies, 328 patients; RR 0.99, 95% CI 0.80 to 1.22, P = 0.89; moderate quality of evidence). There was no evidence for difference in the risks of grade 3/4 cardiac toxicity (one study, 160 patients; RR 0.67, 95% CI 0.11 to 3.88, P = 0.65; low quality of evidence) and other grade 3/4 AEs. None of the studies reported on QoL.
Two RCTs (N = 211) compared IDA with doxorubicin (DOX). Neither study assessed OS. One study showed that there was no evidence for difference in DFS (63 patients; HR 0.62, 95% CI 0.34 to 1.14, P = 0.12; low quality of evidence). The main meta‐analysis for CR rate showed an improved CR rate with IDA (two studies, 187 patients; RR 1.28, 95% CI 1.03 to 1.59, P = 0.02; low quality of evidence). Neither study provided data for the risks of death on induction therapy and relapse. One trial showed that there was no evidence for difference in the risk of grade 3/4 cardiac toxicity (one study, 100 patients; RR 0.31, 95% CI 0.01 to 7.39, P = 0.47; very low quality of evidence). Neither study reported on QoL.
Two RCTs (N = 1037) evaluated IDA versus zorubicin (ZRB). Neither study assessed OS. One trial showed that there was no evidence for difference in DFS (one study, 155 patients; HR 1.25, 95% CI 0.83 to 1.88, P = 0.29; low quality of evidence). The main meta‐analyses for CR and death on induction therapy both showed that there was no evidence for difference (CR rate: two studies, 964 patients; RR 1.04, 95% CI 0.96 to 1.13, P = 0.31; low quality of evidence. risk of death on induction therapy: two studies, 964 patients; RR 0.75, 95% CI 0.50 to 1.13, P = 0.17; moderate quality of evidence). Neither study reported the risks of relapse and grade 3/4 cardiotoxicity. One trial showed that IDA reduced the risk of grade 3/4 mucositis. Neither study reported on QoL.
Authors' conclusions
Compared with DNR in induction therapy of newly diagnosed AML, IDA prolongs OS and DFS, increases CR rate and reduces relapse rate, although increases the risks of death on induction therapy and grade 3/4 mucositis. The currently available evidence does not show any difference between IDA and MIT used in induction therapy of newly diagnosed AML. There is insufficient evidence regarding IDA versus DOX and IDA versus ZRB to make final conclusions. Additionally, there is no evidence for difference on the effect of IDA compared with DNR, MIT, DOX or ZRB on QoL.
Keywords: Humans; Anthracyclines; Anthracyclines/therapeutic use; Antibiotics, Antineoplastic; Antibiotics, Antineoplastic/therapeutic use; Daunorubicin; Daunorubicin/analogs & derivatives; Daunorubicin/therapeutic use; Doxorubicin; Doxorubicin/therapeutic use; Idarubicin; Idarubicin/therapeutic use; Induction Chemotherapy; Induction Chemotherapy/methods; Leukemia, Myeloid, Acute; Leukemia, Myeloid, Acute/drug therapy; Mitoxantrone; Mitoxantrone/therapeutic use; Randomized Controlled Trials as Topic
Plain language summary
Idarubicin for treatment of newly diagnosed acute myeloid leukaemia
Background
Acute myeloid leukaemia (AML) is a type of cancer that mainly affects bone marrow and peripheral blood. Although 40% to 45% of AML patients enjoy long‐term disease‐free survival, most patients will die of the disease. Induction therapy is the first phase of treatment of newly diagnosed AML which is essential for prolonging survival. An anthracycline (a class of chemotherapy drugs derived from the Streptomyces bacterium Streptomyces peucetius var. caesius) combined with cytarabine (a chemotherapy drug used mainly in treatment of haematological malignancies) has remained the standard of induction therapy for several decades. Nowadays there are several kinds of anthracyclines available, among which idarubicin (IDA) draws more attention because of its theoretical advantages in improving efficacy and reducing side effects. However, clinical trials comparing IDA with other anthracyclines have conflicting results.
Objectives
To clarify the role of IDA in induction therapy of newly diagnosed AML.
Methods
Data from available randomised controlled trials (RCTs) that compared IDA with other anthracyclines in induction therapy of newly diagnosed AML were meta‐analysed. The data collected are up to 3 August 2014.
Results
Twenty‐seven RCTs involving 9549 patients were included. The consolidation treatments adopted in the included studies were comparable and had no impact on the results.
Eighteen RCTs assessed IDA versus daunorubicin (DNR; a chemotherapy drug in the anthracycline family). Results showed that IDA compared to DNR prolongs overall survival and disease‐free survival, increases complete remission rate, and reduces relapse rate, although increases the risks of death on induction therapy and grade 3/4 mucositis (a kind of painful inflammation and ulceration of mucous membranes lining the digestive tract). No difference in other various grade 3/4 adverse events was found.
Eight RCTs evaluated IDA versus mitoxantrone (MIT). We found no difference in overall survival, disease‐free survival, complete remission rate, the risks of death on induction therapy and relapse. The risks of various grade 3/4 adverse events were also similar between arms.
Two RCTs compared IDA with doxorubicin (DOX). Results suggested that complete remission rate was improved with IDA. No difference was noted in disease‐free survival and the risk of grade 3/4 cardiac toxicity.
Two other RCTs compared IDA with zorubicin (ZRB). Results suggested that the risk of grade 3/4 mucositis was lower with IDA. No difference was found for disease‐free survival, complete remission rate, the risks of death on induction therapy, grade 3/4 nausea/vomiting, diarrhoea, and hepatic toxicity.
Conclusions
The currently available evidence suggests that in induction therapy of newly diagnosed AML, IDA is superior to DNR in terms of prolonging overall survival and disease‐free survival, increasing complete remission rate and reducing relapse rate, although IDA may increase the risks of death on induction therapy and grade 3/4 mucositis. The current evidence does not support the superiority of IDA over MIT. There is insufficient evidence for clarifying the role of IDA versus DOX or ZRB. Additionally, there is no evidence for a difference on the effect of IDA compared with other anthracyclines (DNR, MIT, DOX and ZRB) on quality of life.
Summary of findings
for the main comparison.
| IDA compared with DNR in induction therapy of patients with newly diagnosed AML | ||||||
|
Patient or population: patients with newly diagnosed AML Settings: inpatients Intervention: IDA Comparison: DNR | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| DNR | IDA | |||||
| Mortality at 2 years | Group with moderate risk1 | HR 0.90 (0.84 to 0.96) | 5976 (12 studies) | ⊕⊕⊕⊕ high | OS is calculated accordingly as mortality | |
| 698 per 1000 | 660 per 1000 (634 to 683) | |||||
| Mortality/relapse at 2 years | Group with moderate risk2 | HR 0.88 (0.81 to 0.96) | 3070 (8 studies) | ⊕⊕⊕⊝ moderate3 | DFS is calculated accordingly as mortality or relapse | |
| 760 per 1000 | 715 per 1000 (685 to 746) | |||||
| Complete remission | Study population | RR 1.04 (1.01 to 1.07) | 6692 (18 studies) | ⊕⊕⊕⊝ moderate3 | ||
| 698 per 1000 | 725 per 1000 (705 to 746) | |||||
| Death on induction therapy | Study population | RR 1.18 (1.01 to 1.36) | 6349 (14 studies) | ⊕⊕⊕⊝ moderate3 | ||
| 90 per 1000 | 106 per 1000 (90 to 122) | |||||
| Relapse | Study population | RR 0.88 (0.80 to 0.98) | 1091 (4 studies) | ⊕⊕⊕⊝ moderate3 | ||
| 550 per 1000 | 484 per 1000 (440 to 539) | |||||
| Grade 3/4 cardiotoxicity | Study population | RR 0.98 (0.70 to 1.37) | 2795 (6 studies) | ⊕⊕⊕⊝ moderate3 | ||
| 46 per 1000 | 45 per 1000 (32 to 63) | |||||
|
Quality of life not reported |
Study population | Not estimable | 0 | See comment | No studies provided data on quality of life | |
| See comment | See comment | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DFS: disease‐free survival; DNR: daunorubicin; HR: hazard ratio; IDA: idarubicin; OS: overall survival;RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The risk for the group with moderate risk was taken from the study with the moderate rate of mortality at two years (Pautas 2010)
2 The risk for the group with moderate risk was taken from the study with the moderate rate of mortality or relapse at seven years (Mandelli 1991)
3Lack of blinding (subjective outcomes are highly susceptible to biased assessment)
2.
| IDA compared with MIT in induction therapy of patients with newly diagnosed AML | ||||||
|
Patient or population: patients with newly diagnosed AML Settings: inpatients Intervention: IDA Comparison: MIT | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| MIT | IDA | |||||
| Mortality at 2 years | Group with moderate risk1 | HR 0.98 (0.89 to 1.08) | 2171 (6 studies) | ⊕⊕⊕⊕ high | OS is calculated accordingly as mortality | |
| 786 per 1000 | 779 per 1000 (746 to 811) | |||||
| Mortality/relapse at 2 years | Group with moderate risk2 | HR 0.88 (0.70 to 1.10) | 249 (4 studies) | ⊕⊕⊝⊝ low3,4 | DFS is calculated accordingly as mortality or relapse | |
| 872 per 1000 | 836 per 1000 (763 to 896) | |||||
| Complete remission | Study population | RR 0.97 (0.92 to 1.03) | 2411 (8 studies) | ⊕⊕⊕⊝ moderate3 | ||
| 669 per 1000 | 649 per 1000 (615 to 689) | |||||
| Death on induction therapy | Study population | RR 1.10 (0.88 to 1.38) | 2055 (5 studies) | ⊕⊕⊕⊝ moderate3 | ||
| 125 per 1000 | 137 per 1000 (110 to 172) | |||||
| Relapse | Study population | RR 0.99 (0.80 to 1.22) | 328 (3 studies) | ⊕⊕⊕⊝ moderate3 | ||
| 494 per 1000 | 489 per 1000 (395 to 603) | |||||
| Grade 3/4 cardiotoxicity | Study population | RR 0.67 (0.11 to 3.88) | 160 (1 study) | ⊕⊕⊝⊝ low3,6 | ||
| 38 per 10005 | 25 per 1000 (4 to 147) | |||||
|
Quality of life not reported |
Study population | Not estimable | 0 | See comment | No studies provided data on quality of life | |
| See comment | See comment | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DFS: disease‐free survival; HR: hazard ratio; IDA: idarubicin; MIT: mitoxantrone; OS: overall survival;RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The risk for the group with moderate risk was taken from the study with the moderate rate of mortality at two years (Archimbaud 1999)
2 The risk for the group with moderate risk was taken from the study with the moderate rate of mortality or relapse at seven years (Archimbaud 1999)
3Lack of blinding (subjective outcomes are highly susceptible to biased assessment)
4Small number of patients
5Obtained from data in Table 5.
6One study only
3.
| IDA compared with DOX in induction therapy of patients with newly diagnosed AML | ||||||
|
Patient or population: patients with newly diagnosed AML Settings: inpatients Intervention: IDA Comparison: DOX | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| DOX | IDA | |||||
|
Mortality at 2 years not reported |
Study population | Not estimable | 0 | See comment | Neither study provided data on overall survival | |
| See comment | See comment | |||||
| Mortality/relapse at 2 years | Study population | HR 0.62 (0.34 to 1.14) | 63 (1 study) | ⊕⊕⊝⊝ low1,2 | DFS is calculated accordingly as mortality or relapse | |
| 914 per 1000 | 782 per 1000 (566 to 939) | |||||
| Complete remission | Study population | RR 1.28 (1.03 to 1.59) | 187 (2 studies) | ⊕⊕⊝⊝ low1,3 | ||
| 573 per 1000 | 733 per 1000 (590 to 911) | |||||
|
Death on induction therapy not reported |
Study population | Not estimable | 0 | See comment | Neither study provided data on death on induction therapy | |
| See comment | See comment | |||||
|
Relapse not reported |
Study population | Not estimable | 0 | See comment | Neither study provided data on relapse | |
| See comment | See comment | |||||
| Grade 3/4 cardiotoxicity | Study population | RR 0.31 (0.01 to 7.39) | 100 (1 study) | ⊕⊝⊝⊝ very low1,3,4 | ||
| 21 per 1000 | 7 per 1000 (0 to 155) | |||||
|
Quality of life not reported |
Study population | Not estimable | 0 | See comment | Neither study provided data on quality of life | |
| See comment | See comment | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DFS: disease‐free survival; DOX: doxorubicin; HR: hazard ratio; IDA: idarubicin; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Lack of blinding (subjective outcomes are highly susceptible to biased assessment)
2One study only
3A large loss to follow‐up in one study
4Relatively few events producing a wide confidence interval around the effect estimate
4.
| IDA compared with ZRB in induction therapy of patients with newly diagnosed AML | ||||||
|
Patient or population: patients with newly diagnosed AML Settings: inpatients Intervention: IDA Comparison: ZRB | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| ZRB | IDA | |||||
|
Mortality at 2 years not reported |
Study population | Not estimable | 0 | See comment | Neither study provided data on overall survival | |
| See comment | See comment | |||||
| Mortality/relapse at 2 years | Study population | HR 1.25 (0.83 to 1.88) | 155 (1 study) | ⊕⊕⊝⊝ low1,2 | DFS is calculated accordingly as mortality or relapse | |
| 478 per 1000 | 556 per 1000 (417 to 705) | |||||
| Complete remission | Study population | RR 1.04 (0.96 to 1.13) | 964 (2 studies) | ⊕⊕⊝⊝ low1,3 | ||
| 686 per 1000 | 714 per 1000 (659 to 775) | |||||
| Death on induction therapy | Study population | RR 0.75 (0.50 to 1.13) | 964 (2 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 100 per 1000 | 75 per 1000 (50 to 113) | |||||
| Relapse not reported | Study population | Not estimable | 0 | See comment | Neither study provided data on relapse | |
| See comment | See comment | |||||
| Grade 3/4 cardiotoxicity not reported | Study population | Not estimable | 0 | See comment | Neither study provided data on grade3/4 cardiotoxicity | |
| See comment | See comment | |||||
|
Quality of life not reported |
Study population | Not estimable | 0 | See comment | Neither study provided data on quality of life | |
| See comment | See comment | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DFS: disease‐free survival; HR: hazard ratio; IDA: idarubicin; RR: risk ratio; ZRB: zorubicin | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Lack of blinding (subjective outcomes are highly susceptible to biased assessment)
2One study only
3Unexplained substantial heterogeneity between studies
Background
Description of the condition
Acute myeloid leukaemia (AML) is a heterogeneous group of clonal malignant myeloid disorders which have clinical similarities but distinct morphologic, immunophenotypic, cytogenetic and molecular features. It is the most common type of myeloid leukaemia with an overall incidence of 3.7 cases per 100,000 persons between 2000 and 2003. The incidence of AML increases with age and the median year at presentation is approximately 65 years (Deschler 2006). AML represents 80% to 90% of acute leukaemia cases in adults but accounts for fewer than 15% of leukaemia cases in children younger than 10 years (Baer 2009). AML is slightly more common among populations of European ethnicity and acute promyelocytic leukaemia (APL), a distinct subtype of AML, has a higher incidence in populations of Latino or Hispanic descent (Douer 1996; Estey 1997).
AML is characterised by an increased number of immature myeloid cells (blasts) in bone marrow, peripheral blood and other tissues, resulting in impaired haematopoiesis (formation of blood cells) manifested by cytopenias (deficiency of specific blood cells) (Lowenberg 1999). It results from genetic alterations in normal haematopoietic stem cells that induce differentiation arrest or excessive proliferation of the affected cells, or both (Jabbour 2006). Several factors have been implied in causing AML which include exposure to ionising radiation, benzene and cytotoxic chemotherapy (Estey 2006). The World Health Organization (WHO) classifies AML into five major categories: (a) AML with recurrent genetic abnormalities; (b) AML with multi‐lineage dysplasia; (c) AML and myelodysplastic syndromes (MDS), therapy‐related; (d) AML not otherwise categorised; and (e) acute leukaemia of ambiguous lineage (Baer 2009). Typical clinical presentations of AML are fatigue and weakness, haemorrhage, or infections and fever due to decreases in red blood cells, platelets or white blood cells, respectively. Additionally, leukaemic infiltration of various tissues can produce a variety of corresponding symptoms such as enlarged liver (hepatomegaly), enlarged spleen (splenomegaly), enlarged lymph nodes (lymphadenopathy), leukaemia cutis (the outermost, nonvascular layer of the skin) and so on (Lowenberg 1999). Besides these common clinical presentations of AML, acute promyelocytic leukaemia (APL) possesses additional characteristics. It used to be considered the most fatal subtype of AML because of potential fatal haemorrhage due to consumptive coagulopathy. However, it is now regarded as the most curable subtype due to its high sensitivity to all‐trans retinoic acid (ATRA) and arsenic trioxide (ATO) (Wang 2008).
Studies on the pathogenesis and prognosis of AML have made revolutionary progress; however, the treatment for AML remains unsatisfactory. Only 40% to 45% of AML patients enjoy long‐term disease‐free survival (DFS) and most patients still die of their disease, primarily due to persistent or relapsed AML (Burnett 2011). New progress in the treatment of AML is required.
Description of the intervention
Treatment of AML consists of two phases: remission‐induction therapy phase and post‐remission therapy phase. The former aims to attain a complete remission (CR), while the latter aims to maintain the CR. Achieving CR by remission‐induction therapy is essential for prolonging survival and obtaining a cure for AML patients. For several decades, a combination of an anthracycline (a class of chemotherapy drugs derived from the Streptomyces bacterium Streptomyces peucetius var. caesius) and cytarabine (Ara‐C) has been the standard for remission‐induction therapy of AML (Dohner 2010). Therefore, selecting the most effective and tolerable anthracycline is key to maximising treatment outcomes.
Daunorubicin (DNR) is the most widely used anthracycline. The standard dose of DNR used in remission‐induction therapy is 45 mg/m²/d for three days (Fernandez 2009). A combination of three days of DNR at a dose of 40 mg/m²/d to 60 mg/m²/d and seven days of Ara‐C at a dose of 100 mg/m²/d to 200 mg/m²/d generally has been used for more than 40 years (Burnett 2011; Lowenberg 1999). With this regimen, approximately 60% to 80% of adults with AML achieve CR, whereas only 40% to 45% of patients enjoy long‐term DFS (Burnett 2011; Lowenberg 1999; Tallman 2005; Zittoun 1995). Additionally, DNR tends to cause serious cumulative injury to the heart resulting in congestive cardiomyopathy and, ultimately, congestive heart failure, which is usually refractory to medical therapy. Other common side effects include myelosuppression, nausea, vomiting, diarrhoea, alopecia and mucositis (Hande 2009).
To improve the efficacy and reduce the side effects of remission‐induction therapy, various alternative anthracyclines were developed and introduced into clinics in the 1980s, among which idarubicin (IDA) is a most promising one (Johnson 1998). IDA, also called 4'‐demethoxydaunorubicin (4‐DMDR), is a DNR derivative synthesised by replacing the C‐4 methoxyl group with a hydrogen atom (Arcamone 1976). With this minor structural alteration, IDA has several theoretical advantages over the parent compound: (1) IDA has a more effective antileukaemia activity (Casazza 1980); (2) IDA is active by both intravenous and oral routes of administration (Ganzina 1986); (3) IDA has an ability to overcome the multidrug resistant (MDR) phenotype and reduces the development of drug resistance (Berman 1992); (4) IDA is less cardiotoxic and is well tolerated (Cersosimo 1992). IDA was registered and approved by the Food and Drug Administration (FDA) of USA in 1990. The standard dose of IDA used in remission‐induction therapy is 12 mg/m²/d for three days (Ohtake 2011). At present, IDA has been used as the first‐line therapy at a dose of 10 mg/m²/d to 12 mg/m²/d for three days in younger adult patients (18 to 60 years) with newly diagnosed AML, or relapsed/refractory AML (Dohner 2010).
How the intervention might work
IDA is an anthracycline antineoplastic agent. It mediates control of AML by two molecular mechanisms. First, IDA inhibits DNA topoisomerase II, which is a nuclear enzyme that modulates DNA topology by passing a double‐stranded DNA through a transient break in the DNA backbone. By poisoning the enzyme to prevent it from re‐ligating (i.e. binding back together) cleaved DNA, IDA converts topoisomerase II into a toxin, resulting in high levels of transient protein‐associated breaks in the genome of treated cells. Second, IDA intercalates into base pairs of DNA and generates free radicals to break the DNA strand. Both eventually lead to the death of leukaemia cells (Hande 2009). Experimental laboratory studies have indicated that IDA and DNR have equal affinity for DNA and comparable inhibitory effects on DNA topoisomerase II (Ganzina 1986). The higher antileukaemia activity of IDA may result from its metabolite idarubicinol, which is more active and has a longer half‐life than the metabolite of DNR (Robert 1992). For the ability of overcoming the MDR phenotype, some studies suggest that IDA has a high lipophilic (having an affinity for, tending to combine with, or capable of dissolving in lipids) coefficient and is less of a substrate for P‐glycoprotein (P‐gp) than DNR, which acts as an active efflux pump, thereby allowing for greater intracellular drug accumulation (Berman 1992; Supino 1977).
A great number of phase I/II trials support the activity of IDA in AML. In phase I trials, IDA was demonstrated to be less cardiotoxic and the dose‐limiting toxicity of the drug was myelosuppression (Berman 1983;Kaplan 1982). In later phase II trials, as a single agent, IDA induced CR in about 20% of adult patients with relapsed or refractory AML (Carella 1984; Hayat 1984). Combining IDA with Ara‐C increased CR to a range of 24% to 70% in similar groups of heavily pre‐treated patients (Berman 1989; Harousseau 1987; Lambertenghi‐Deliliers 1987). In previously untreated AML, more than 80% of patients achieved CR after being treated with a combination of IDA, Ara‐C and etoposide (an anti‐cancer agent which kills cancer cells by inhibiting their DNA synthesis) (Carella 1987). For newly diagnosed APL, IDA, when combined with ATRA, induced a CR rate higher than 80% either in adults or in elderly patients (Avvisati 1996; Latagliata 1997).
On the basis of these trials, numerous prospective, randomised controlled trials (RCTs) testing the superiority of IDA versus other anthracyclines including DNR have been conducted in previously untreated AML patients (Beksac 1998; Berman 1991; Creutzig 2001; Harousseau 1996; Indrak 2001; Mandelli 1991; Mandelli 2009; Ohtake 2011; Pignon 1996; Reiffers 1996; Rowe 2004; Vogler 1992; Wiernik 1992). However, the outcomes of these RCTs are inconsistent. Three initial RCTs comparing standard dose IDA (12/13 mg/m²/d for three days) with standard dose DNR (45/50 mg/m²/d for three days) reported a superior CR rate for the IDA group (Berman 1991; Vogler 1992; Wiernik 1992). However, the long‐term follow‐up of the three RCTs revealed that the IDA group had a better overall survival (OS) than the DNR group in only one of the three RCTs (Berman 1997). A study published by Mandelli in 1991, which compared IDA (12 mg/m²/d for three days) with DNR (45 mg/m²/d for three days) in elderly AML patients (age greater than 55 years), failed to demonstrate any significant difference in CR rate, OS and relapse‐free survival (RFS) between the two arms (Mandelli 1991). In another study published by Mandelli in 2009, the use of IDA (10 mg/m²/d for three days) was superior to DNR (50 mg/m²/d for three days) in terms of DFS, survival from CR and OS, but was similar to mitoxantrone (12 mg/m²/d for three days) (Mandelli 2009). Moreover, in two recent studies, doubling the dose of DNR from the standard dose (45 mg/m²/d for three days) to 90 mg/m²/d for three days significantly improved the CR rate and duration of OS (Fernandez 2009; Lowenberg 2009). In a more recent study conducted by Ohtake, DNR at a dose of 50 mg/m²/d for five days was found to be equivalent to IDA (12 mg/m²/d for three days) in CR rate, RFS and OS without increasing the risk of infection or cardiomyopathy (Ohtake 2011). Therefore, the superiority of IDA versus other anthracyclines remains a matter of debate.
Why it is important to do this review
Anthracyclines have been the core treatment for AML for several decades; thus, selecting the most effective and tolerable anthracycline is key to maximising treatment outcomes. In spite of the theoretical advantages of IDA, RCTs comparing induction therapy based on IDA with those based on other anthracyclines have conflicting results. There is no evidence that it would definitively prove the superiority of IDA over other anthracyclines with respect to CR rate, RFS, DFS and OS. We would like to assess which anthracycline is the most effective to be used for induction therapy. Although a meta‐analysis for IDA is available (AML Collaborative Group 1998), it only included RCTs published before 1996 and many new RCTs have been published since then (Beksac 1998; Creutzig 2001; Indrak 2001; Mandelli 2009; Morita 2010; Ohtake 2011; Rowe 2004). It is important to update the information by including all new trials. Therefore, we undertook this systematic review to obtain definitive evidence on the role of IDA versus other anthracyclines in the treatment of AML. Our review informed about the current status of clinical practice and provided some guidance for future clinical studies in this area.
Objectives
To assess the effects of idarubicin (IDA) versus other anthracyclines for patients with newly diagnosed acute myeloid leukaemia (AML) in induction therapy.
Methods
Criteria for considering studies for this review
Types of studies
This review is referring to the already published protocol (Li 2013).
We accepted only randomised controlled trials (RCTs) in this review and we included both full‐text and abstract publications, irrespective of publication language. We excluded quasi‐randomised trials and cross‐over trials due to the risk of bias.
Types of participants
Participants were patients with newly diagnosed AML according to French‐American‐British (FAB) (Bennett 1976) or WHO diagnostic criteria (Vardiman 2009), or both, irrespective of age, gender and ethnicity. For studies with mixed populations, we included data from the AML subgroups. We excluded two studies because subgroup data for newly diagnosed AML patients were not available and less than 80% of the patients had newly diagnosed AML (Belhabri 1999; Morita 2010).
Types of interventions
Experimental intervention
Induction therapy based on IDA: any form of application, any dose.
Control intervention
Induction therapy based on other anthracyclines, e.g. daunorubicin (DNR), doxorubicin (DOX), aclarubicin (ACR) and mitoxantrone (MIT): any form of application, any dose.
Drugs combined with IDA or other anthracyclines had to be identical between arms.
Types of outcome measures
Primary outcomes
Overall survival (OS): defined as the time from randomisation or entry into study to death from any cause or last follow‐up.
Disease‐free survival (DFS): defined as the time from complete remission (CR) to first relapse, or death from any cause or the last follow‐up.
Secondary outcomes
Complete remission (CR): defined by bone marrow blasts < 5%, no blasts in peripheral blood, absence of extramedullary disease, absolute neutrophil count > 1.0 x 109/L, platelet count > 100 x 109/L and independence of red cell transfusions (Dohner 2010).
Death on induction therapy.
Relapse: defined by recurrence of bone marrow blasts > 5%, or reappearance of blasts in the blood, or development of extramedullary disease (Dohner 2010).
Adverse events (AEs) (haematologic toxicity including mean duration with a neutrophil count < 1.0 x 109/L, mean duration with a platelet count < 100 x 109/L and median duration of hospitalisation days after induction treatment; non‐haematologic toxicity including grade 3/4 toxicity of nausea/vomiting, alopecia, diarrhoea, mucositis, hepatic, renal or cardiac dysfunction, etc.).
Quality of life (QoL).
Search methods for identification of studies
We adopted search strategies from those suggested in chapter six of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). We applied no language restriction to reduce language bias.
Electronic searches
We searched the following bibliographic databases.
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2014, Issue 8) (see Appendix 1).
Ovid MEDLINE (1946 to 3 August 2014) (see Appendix 2).
EMBASE (1974 to 3 August 2014) (see Appendix 3).
Chinese BioMedical Literature Database (CBM) (1978 to 3 August 2014) (see Appendix 4).
We searched conference proceedings of the following societies for the years that were not included in CENTRAL (see Appendix 5).
American Society of Clinical Oncology (ASCO) (1990 to 2014) (available at: http://www.asco.org/).
American Society of Hematology (ASH) (2000 to 2014) (available at: http://www.hematology.org/).
European Hematology Association (EHA) (2000 to 2014) (available at: http://www.ehaweb.org/).
European Society of Medical Oncology (ESMO) (2000 to 2014) (available at: http://www.esmo.org/).
We searched the following database of ongoing studies.
Metaregister of controlled trials (available at: http://www.controlled‐trials.com/mrct/) (see Appendix 5).
Searching other resources
We handsearched:
Data collection and analysis
Selection of studies
Two review authors (XL, SX) independently screened all the obtained titles and abstracts from the above‐mentioned resources and rejected studies that were obviously irrelevant. We made our best efforts to obtain the full texts of all potentially relevant studies. Then, the two review authors independently screened the full texts of the studies with the inclusion criteria stated in the section Criteria for considering studies for this review. The review authors were not blind to the study authors' names, institutions and journal of publication. We resolved any disagreement through discussion. According to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses), we documented the overall number of studies identified, included and excluded, and the reasons for exclusions at every stage of searching and screening of the literature in a flow diagram (Liberati 2009; Moher 2009).
Data extraction and management
Two review authors (XL, SX) independently extracted data from the included studies according to chapter seven of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We resolved disagreements by consensus. We recorded all the extracted data on paper data collection forms and entered them into Review Manager 5 (RevMan 2014). We extracted the following groups of data.
Quality assessment: sequence generation, allocation sequence concealment, blinding, incomplete outcome data, selective outcome reporting, and other concerns about bias.
Study characteristics: title, first author, contact address, publication data, publication status (published, published as abstract or unpublished), duplicate publications, country, language, trial design, aims, setting (inpatients or outpatients), data (defined as recruitment initiation year), centre (single centre or multicentre), trial sponsor, inclusion/exclusion criteria, reasons for exclusion, sample size, power calculation, comparability of groups, subgroup analysis, stopping rules described, duration of follow‐up, results, and conclusion.
Participant characteristics: age, gender, ethnicity, Eastern Cooperative Oncology Group (ECOG) status, total number recruited/randomised/analysed, FAB subtype (M0‐M7, not assessed), cytogenetics (favourable, intermediate, adverse, not assessed), treatment history, additional diagnoses, lost to follow‐up numbers, and dropouts (percentage in each arm) with reasons.
Interventions: experimental and control interventions, time, dosage, regimen, cycles and route of interventions, compliance to interventions, additional interventions given, and any differences between interventions.
Results of outcomes: overall survival (OS) (hazard ratio (HR); 95% confidence interval (CI)/P value), disease‐free survival (DFS) (HR; 95% CI/P value), complete remission (CR), death on induction therapy, relapse, adverse events and quality of life (QoL).
Whenever possible, we sought missing data from the authors of studies.
Assessment of risk of bias in included studies
Two review authors (XL, SX) independently assessed quality and risk of bias for each included study. According to the recommendations in chapter eight of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b), we used a questionnaire for the following criteria.
Sequence generation.
Allocation concealment.
Blinding (participants, personnel, outcome assessors).
Incomplete outcome data.
Selective outcome reporting.
Other sources of bias.
Our judgement of the review involved an answer for each criterion based on a three‐point scale (low risk of bias, high risk of bias and unclear) and a summary description. We resolved disagreements between the two review authors by consensus.
Measures of treatment effect
For dichotomous outcome data, we counted attainment of CR as an event for 'CR'; death due to hypoplastic marrow or progressive disease, or death before marrow re‐evaluation for 'death on induction therapy'; occurring of relapse for 'relapse'; and development of adverse events for 'adverse events'.
For dichotomous data, we calculated risk ratio (RR) as a measure of treatment effect with 95% CIs as a measure of uncertainty. For time‐to‐event data, we calculated HR with 95% CIs using the methods described by Parmar (Parmar 1998) and Tierney (Tierney 2007). For continuous data (QoL scales), we planned to estimate standardised mean difference (SMD) with 95% CIs.
Unit of analysis issues
For parallel group designed RCTs in which participants were individually randomised to one of two intervention groups, and a single measurement for each outcome from each participant was collected and analysed, we used the individual participant as a unit of analysis.
For RCTs with three arms, we combined the two comparators together in a 1:2 comparison, rather than including each comparison idarubicin (IDA) to control separately (to avoid double counting the IDA patients).
Dealing with missing data
We dealt with the missing data as suggested in chapter 16 of theCochrane Handbook for Systematic Review of Intervention (Higgins 2011c) and the National Research Council (NRC) report on missing data (Little 2012). Firstly, we documented the reasons why data were missing as clearly as possible. Then, we decided on a primary assumption about the missing‐data mechanism, including "missing at random" and "missing not at random". Because we judged all missing data as "missing at random", we analysed the only available data (i.e. ignoring the missing data).
In the event that we assumed data not to be 'missing at random', we planned to input the missing data with replacement values and treat these as if they were observed (e.g. last observation carried forward, imputing an assumed outcome such as assuming all were poor outcomes, imputing the mean, imputing based on predicted values from a regression analysis).
Assessment of heterogeneity
We detected heterogeneity of treatment effects across studies using the Chi² test with a significance level at P values < 0.1. We also used the I² statistic for quantifying inconsistency (Deeks 2011). We used the following rough guide for the interpretation of I².
0% to 40%: low heterogeneity.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: considerable heterogeneity.
We explored potential causes of heterogeneity by sensitivity and subgroup analysis as defined below.
Assessment of reporting biases
We made our best efforts to minimise the impact of reporting biases by searching comprehensively for studies that met the eligibility criteria for the review, including unpublished studies and trial registries, making no restriction on location or language, and carefully examining the author, institute and detailed information of studies. When at least 10 studies were included in the meta‐analysis, we assessed the possibility of publication bias using a funnel plot (Sterne 2011).
Data synthesis
We performed data analyses according to the recommendations of chapter nine of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). We used the Cochrane statistical package Review Manager 5 (RevMan 2014) to synthesise data. One review author (XL) inputted data into the software and a second review author (SX) checked it for accuracy. We firstly adopted a fixed‐effect model for meta‐analyses. We also carried out a random‐effects analysis in terms of sensitivity analyses. We chose the Mantel‐Haenszel method for dichotomous data outcomes and the inverse variance method for time‐to‐event data. Had we identified any continuous data outcomes, we would have used the inverse variance method.
We used the GRADE tool (GRADEpro 2011) to assess the level of evidence and to create 'Summary of Finding' tables for each comparison including IDA versus DNR, IDA versus MIT, IDA versus doxorubicin (DOX) and IDA versus zorubicin (ZRB). The outcomes of OS, DFS, CR, death on induction therapy, relapse, grade 3/4 cardiotoxicity and QoL were included in each 'Summary of Finding' table. We assessed the quality of the body of evidence with the GRADE approach (GRADE Working Group 2004).
Subgroup analysis and investigation of heterogeneity
The following subgroup analysis was planned in the protocol, but in the end was not conducted.
Anthracycline agent types of control intervention (DNR, DOX, ACR, or MIT).
To avoid clinical heterogeneity, we did not perform subgroup analysis by anthracycline agent types of control intervention but carried out separate meta‐analyses for IDA versus different anthracyclines.
We conducted the following subgroup analyses in the systematic review.
Dose of IDA (8 mg/m²/d, 10 mg/m²/d, 12 mg/m²/d, or other doses used).
Total dose of DNR (< 180 mg/m² or ≥ 180 mg/m²).
Different dose of IDA versus different dose of DNR (8 mg/m²/d IDA versus 45 mg/m²/d DNR, 8 mg/m²/d IDA versus 60 mg/m²/d DNR, 8 mg/m²/d IDA versus 90 mg/m²/d DNR, 10 mg/m²/d IDA versus 45 mg/m²/d DNR, 10 mg/m²/d IDA versus 60 mg/m²/d DNR, 10 mg/m²/d IDA versus 90 mg/m²/d DNR, 12 mg/m²/d IDA versus 45 mg/m²/d DNR, 12 mg/m²/d IDA versus 60 mg/m²/d DNR, 12 mg/m²/d IDA versus 90 mg/m²/d DNR, or other doses used).
Cytogenetic risk stratification (favourable, intermediate, or adverse).
Age (< 15 years or ≥ 15 years to < 60 years or ≥ 60 years).
AML subtypes (APL or other subtypes of AML).
We assessed differences between subgroups using the Chi² test with a significance level at P value < 0.05 (Deeks 2001).
Sensitivity analysis
We performed sensitivity analysis based on:
fixed‐effect versus random‐effects models;
methodological quality of the studies (including versus excluding studies with high risk of bias).
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
Results of the search
A total of 2017 potentially relevant references were identified through database searches and handsearching. After removing duplicates, 1547 references were screened. Of these, 1462 were excluded at the initial stage of screen. The remaining references were retrieved as full‐text publications or abstract publications for further evaluation. Of these publications, 25 publications were excluded and 27 included studies (59 publications) and one ongoing study (one publication) that fulfilled our pre‐defined inclusion criteria were identified. The overall number of references screened, identified, selected, excluded and included is documented according to the PRISMA flow diagram (Figure 1).
1.
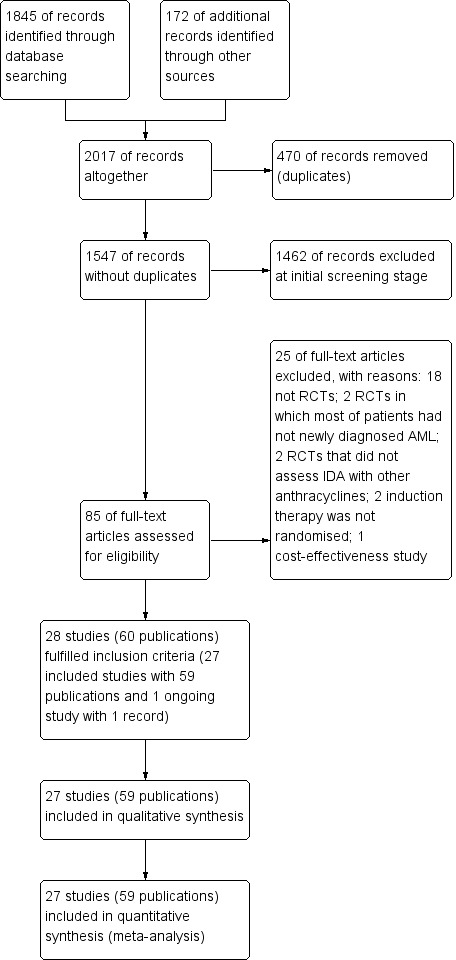
Study flow diagram.
Included studies
Twenty‐seven studies in 59 publications with 9549 patients were included in the systematic review (Archimbaud 1999; Beksac 1998; Berman 1991; Bezwoda 1990; Creutzig 2001; Creutzig 2013; De Moerloose 2011; Eridani 1989; Feng 2010; Gardin 2007; Harousseau 1996; Indrak 2001; Intragumtornchai 1999; Jia 2011; Lee 2012; Mandelli 1991; Mandelli 2009; Masaoka 1996; Ohtake 2011; Pautas 2010; Pignon 1996; Récher 2014; Reiffers 1996; Rowe 2004; Vogler 1992; Wang 2011; Wiernik 1992). The characteristics of included studies are summarised in the table Characteristics of included studies. With the exception of two studies (Intragumtornchai 1999; Masaoka 1996), which did not provide dates on study recruitment, the other studies recruited patients between the years 1984 to 2012. The median follow‐up period ranged from 9.5 to 118.8 months. All but seven studies (Beksac 1998; Berman 1991; Bezwoda 1990; Feng 2010; Intragumtornchai 1999; Jia 2011; Wang 2011) were multicentre studies. Three studies (Feng 2010; Jia 2011; Wang 2011) were published in Chinese, one study was published (Indrak 2001) in Czech, and the other studies were published in English. Two review authors independently extracted data from full‐text publications for 24 studies and from abstract publications for the other three studies (De Moerloose 2011; Intragumtornchai 1999; Lee 2012).
Design
Twenty‐two included studies were two‐armed randomised controlled trials (RCTs) and the other five studies (Beksac 1998; Feng 2010; Mandelli 2009; Pautas 2010; Rowe 2004) were three‐armed RCTs.
Sample size
The smallest study (Eridani 1989) randomised 24 patients and the largest one (Mandelli 2009) randomised 2157 patients.
Location
Six included studies were conducted in France (Gardin 2007; Harousseau 1996; Pautas 2010; Pignon 1996; Récher 2014; Reiffers 1996). Three studies were conducted in the US (Berman 1991; Vogler 1992; Wiernik 1992) and another three were conducted in China (Feng 2010; Jia 2011; Wang 2011). Two studies were conducted in Janpan (Masaoka 1996; Ohtake 2011). One study recruited patients in Belgium (De Moerloose 2011), one in the UK (Eridani 1989), one in Italy (Mandelli 1991), one in South Africa (Bezwoda 1990), one in South Korea (Lee 2012), one in Czech Republic (Indrak 2001), and one in Turkey (Beksac 1998). Five studies were multinational studies: two recruited patients in Europe (Archimbaud 1999; Mandelli 2009), one in Germany, Austria, Switzerland, and the Czech Republic (Creutzig 2013), one in Germany, Austria and Switzerland (Creutzig 2001), and another one in Israel and the US (Rowe 2004). One study did not report the country of recruitment (Intragumtornchai 1999).
Participants
A total of 9549 male and female patients with newly diagnosed AML defined by the FAB (Bennett 1976) or WHO criteria (Vardiman 2009) were randomised. One study included both patients with newly diagnosed AML and patients with acute lymphocytic leukaemia (ALL), but subtype data for patients with newly diagnosed AML were available (Jia 2011). Ten studies only included patients with non‐M3‐AML (De Moerloose 2011; Feng 2010; Gardin 2007; Intragumtornchai 1999; Lee 2012; Mandelli 2009; Ohtake 2011; Pautas 2010; Récher 2014; Wang 2011), and the other studies included patients with any subtype of AML. Median age of the patients at study entry ranged between eight and 69 years (range 0 to 86 years).
Intervention
Fourteen studies evaluated idarubicin (IDA) versus daunorubicin (DNR) in induction therapy of patients with newly diagnosed AML. The baseline chemotherapy was either IDA or DNR plus cytarabine (Ara‐C) in 12 studies (Berman 1991; Eridani 1989; Gardin 2007; Jia 2011; Lee 2012; Mandelli 1991; Masaoka 1996; Ohtake 2011; Récher 2014; Reiffers 1996; Vogler 1992; Wiernik 1992), and it was either IDA or DNR plus Ara‐C and etoposide in the other two studies (Creutzig 2001; Creutzig 2013). The total dose of IDA ranged from 24 mg/m² to 40 mg/m², and the total dose of DNR ranged from 75 mg/m² to 270 mg/m².
Four studies assessed the role of IDA versus mitoxantrone (MIT) in induction therapy of patients with newly diagnosed AML. The baseline chemotherapy was either IDA or MIT plus Ara‐C in two studies (Indrak 2001; Wang 2011), and it was either IDA or MIT plus Ara‐C and etoposide in the other two studies (Archimbaud 1999; De Moerloose 2011). The total dose of IDA ranged from 24 mg/m² to 36 mg/m², and MIT ranged from 18 mg/m² to 30 mg/m².
Two studies compared the effects of IDA with doxorubicin (DOX) in induction therapy of patients with newly diagnosed AML. The baseline chemotherapy was either IDA or DOX plus Ara‐C in both studies (Bezwoda 1990; Intragumtornchai 1999). In Bezwoda 1990, IDA was administrated orally and its total dose was 60 mg/m². In Intragumtornchai 1999, the total dose of IDA was 36 mg/m². The total dose of DOX was 90 mg/m² in the two studies.
Two studies assessed the effects of IDA versus zorubicin (ZRB) in induction therapy of patients with newly diagnosed AML (Harousseau 1996; Pignon 1996). The baseline chemotherapy was either IDA or ZRB plus Ara‐C in both studies (Harousseau 1996; Pignon 1996). The total doses of IDA and ZRB were identical between the two studies, i.e., 40 mg/m² and 800 mg/m², respectively.
Five studies were three‐armed RCTs (Beksac 1998; Feng 2010; Mandelli 2009; Pautas 2010; Rowe 2004). Of these, four studies compared the effects of IDA with DNR and MIT (Beksac 1998; Feng 2010; Mandelli 2009; Rowe 2004). In Feng 2010 and Rowe 2004, IDA or DNR or MIT was combined with Ara‐C; in Mandelli 2009, IDA or DNR or MIT was combined with Ara‐C and etoposide. We respectively extracted two comparisons of IDA versus DNR and IDA versus MIT for the three studies. In Beksac 1998, both IDA and MIT were combined with Ara‐C, but DNR was combined with Ara‐C and etoposide. Therefore, we only included the comparison of IDA verses MIT for this study. One other study evaluated 36 mg/m² IDA versus 48 mg/m² IDA versus DNR (Pautas 2010), and both IDA and DNR were combined with Ara‐C. For this study, we combined the 36 mg/m² IDA group and the 48 mg/m² IDA group to create a single pair‐wise comparison of IDA versus DNR (Higgins 2011c). The total dose of IDA ranged from 24 mg/m² to 50 mg/m², DNR ranged from 120 mg/m² to 240 mg/m² and MIT ranged from 24 mg/m² to 36 mg/m².
After achieving CR with induction therapy, 12 studies (Archimbaud 1999; Beksac 1998; Creutzig 2001; De Moerloose 2011; Eridani 1989; Indrak 2001; Lee 2012; Mandelli 1991; Mandelli 2009; Pignon 1996; Récher 2014; Rowe 2004) reported that patients received the same consolidation therapy, six studies (Berman 1991; Bezwoda 1990; Pautas 2010; Reiffers 1996; Vogler 1992; Wiernik 1992) adopted a consistent drug in consolidation therapy as in induction therapy, four studies (Creutzig 2013; Gardin 2007; Harousseau 1996; Ohtake 2011) re‐randomised patients, five studies (Feng 2010; Intragumtornchai 1999; Jia 2011; Masaoka 1996; Wang 2011) that had not reported the method of consolidation therapy only provided results on CR and adverse events (AEs).
Outcomes
Primary outcome measures
Overall survival (OS) data were available from 16 studies (Archimbaud 1999; Beksac 1998; Berman 1991; Creutzig 2013; De Moerloose 2011; Gardin 2007; Indrak 2001; Mandelli 1991; Mandelli 2009; Ohtake 2011; Pautas 2010; Récher 2014; Reiffers 1996; Rowe 2004; Vogler 1992; Wiernik 1992).
Disease‐free survival (DFS) data were available from 13 studies (Archimbaud 1999; Beksac 1998; Bezwoda 1990; Indrak 2001; Mandelli 1991; Mandelli 2009; Ohtake 2011; Pignon 1996; Récher 2014; Reiffers 1996; Rowe 2004; Vogler 1992; Wiernik 1992).
Secondary outcome measures
All studies reported complete remission (CR) data.
Twenty studies assessed death on induction therapy (Archimbaud 1999; Beksac 1998; Berman 1991; Creutzig 2001; Creutzig 2013; De Moerloose 2011; Eridani 1989; Gardin 2007; Harousseau 1996; Mandelli 1991; Mandelli 2009; Ohtake 2011; Pautas 2010; Pignon 1996; Récher 2014; Reiffers 1996; Rowe 2004; Vogler 1992; Wang 2011; Wiernik 1992).
Seven studies provided relapse data (Archimbaud 1999; Beksac 1998; Creutzig 2013; De Moerloose 2011; Pautas 2010; Reiffers 1996; Vogler 1992).
Eighteen studies mentioned various grade 3/4 adverse events (AEs) (Archimbaud 1999; Beksac 1998; Berman 1991; Bezwoda 1990; Creutzig 2001; Creutzig 2013; De Moerloose 2011; Mandelli 1991; Mandelli 2009; Masaoka 1996; Ohtake 2011; Pautas 2010; Pignon 1996; Récher 2014; Reiffers 1996; Vogler 1992; Wang 2011; Wiernik 1992).
None of the studies provided data regarding quality of life (QoL).
Conflict of interest
In six studies, the authors declared no potential conflict of interest (De Moerloose 2011; Gardin 2007; Mandelli 2009; Ohtake 2011; Pautas 2010; Récher 2014). In Creutzig 2013, an author was a member of the advisory board from Galen, and the other authors declared no potential conflict of interest. In Eridani 1989 and Mandelli 1991, IDA was supplied by Farmitalia‐Carlo Erba Research Laboratories, Milan, Italy. All other studies did not provide a conflict of interest statement.
Eleven studies stated that they received grants from non‐pharmaceutical organizations (Beksac 1998; Creutzig 2001; Creutzig 2013; De Moerloose 2011; Gardin 2007; Harousseau 1996; Mandelli 2009; Ohtake 2011; Pautas 2010; Récher 2014; Rowe 2004). Three studies were funded in part by Adria Laboratories, the manufacturer of IDA (Berman 1991; Vogler 1992; Wiernik 1992). The other studies did not provide information of funding source.
Excluded studies
Twenty‐five articles were excluded after detailed evaluation of full‐text publications for the following reasons:
non‐randomised studies (Buchner 2012; Candoni 2009; Chan‐Lam 1992; Creutzig 2000; Creutzig 2005; Dluzniewska 2005; Gardin 2013; Keldsen 1990; Lambertenghi‐Deliliers 1989; Leone 1999; Li 2013a; O'Brien 2002; Oriol 2003; Reinhardt 2005; Shi 2013; Volkova 1993; Wheatley 2001; Xia 2013);
randomised studies with a low number of newly diagnosed AML patients; subtype data for them were not available (Belhabri 1999; Morita 2010);
randomised studies that did not assess IDA versus other anthracyclines (Castaigne 2004; Liu 2005);
no randomisation to induction therapy (Lange 2008; Witz 1995);
cost‐effectiveness study (Pashko 1991).
See also the table Characteristics of excluded studies.
Risk of bias in included studies
Overall, the risk of bias of the included studies was unclear to high. See: Characteristics of included studies, 'Risk of bias' tables; Figure 2 and Figure 3.
2.
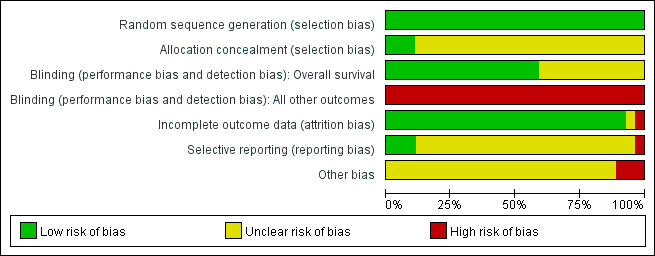
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
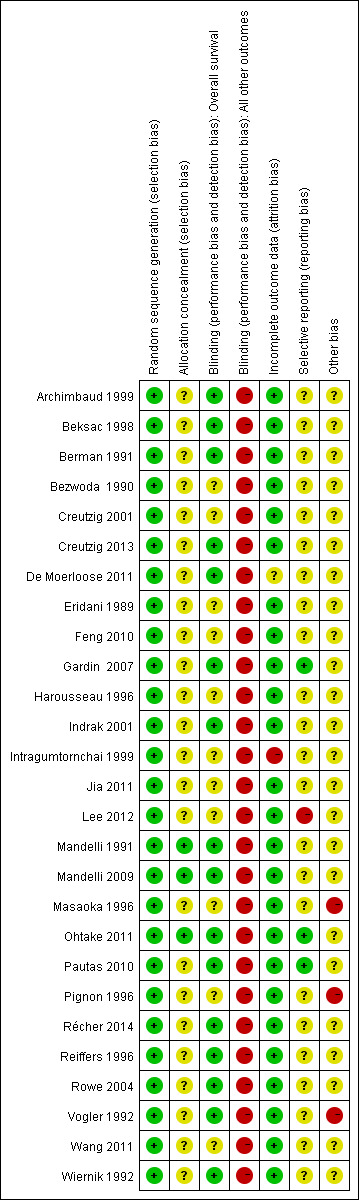
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All included studies stated that they were 'randomised'. One study stated that random sequence was generated by referring to a random number table (Jia 2011). We judged the study as low risk of bias for random sequence generation. The other studies did not report the method of random sequence generation but they probably had an adequate sequence generation because they did not explicitly state they were quasi‐randomised, or were very old. We also judged these studies as low risk of bias for the domain. Allocation was adequately concealed in three studies (central allocation) (Mandelli 1991; Mandelli 2009; Ohtake 2011) so they were judged as low risk of bias for allocation concealment; no information was available for the other studies and they were judged as unclear risk of bias for the domain.
Blinding
Patients and physicians were not blinded in five included studies (Gardin 2007; Lee 2012; Ohtake 2011; Pautas 2010; Récher 2014); no information was available for the other studies. None of the studies provided information for blinding of the outcome assessors.
Overall survival (OS)
Because to define the status of a patient as dead or alive is not influenced by knowledge of the assigned intervention (Higgins 2011b), the potential risk of bias for blinding regarding OS was judged as 'low' in the 16 studies that reported this outcome (Archimbaud 1999; Beksac 1998; Berman 1991; Creutzig 2013; De Moerloose 2011; Gardin 2007; Indrak 2001; Mandelli 1991; Mandelli 2009; Ohtake 2011; Pautas 2010; Récher 2014; Reiffers 1996; Rowe 2004; Vogler 1992; Wiernik 1992). For the 11 studies that did not report OS data (Bezwoda 1990; Creutzig 2001; Eridani 1989; Feng 2010; Harousseau 1996; Intragumtornchai 1999; Jia 2011; Lee 2012; Masaoka 1996; Pignon 1996; Wang 2011), the judgment was classified as unclear risk of bias.
All other outcomes
As knowledge of the assigned intervention will impact on all other outcomes except OS, all studies were judged as high risk of bias for the domain of blinding regarding all other outcomes.
Incomplete outcome data
Fifteen studies had no missing outcome data and included all randomised patients in the analysis (Archimbaud 1999; Creutzig 2001; Creutzig 2013; Eridani 1989; Feng 2010; Gardin 2007; Indrak 2001; Jia 2011; Lee 2012; Mandelli 1991; Mandelli 2009; Ohtake 2011; Reiffers 1996; Wang 2011; Wiernik 1992). We judged these studies as low risk of attrition bias. Eleven studies reported missing outcome data (Beksac 1998; Berman 1991; Bezwoda 1990; Harousseau 1996; Intragumtornchai 1999; Masaoka 1996; Pautas 2010; Pignon 1996; Récher 2014; Rowe 2004; Vogler 1992). Reasons for missing data and their treatment allocation were given in eight studies (Beksac 1998; Bezwoda 1990; Masaoka 1996; Pautas 2010; Pignon 1996; Récher 2014; Rowe 2004; Vogler 1992). We judged the eight studies as low risk of attrition bias. Two studies gave reasons for missing data without their allocation (Berman 1991; Harousseau 1996). As the percentage of missing data was less than 10% of randomised patients, we judged these two studies as low risk of attrition bias.
In another study, the proportion of missing outcomes reached 19% (Intragumtornchai 1999) of randomised patients and we judged the study as high risk of attrition bias. In one study published as abstracts only (De Moerloose 2011), there was insufficient information about statistical methods and patient analyses, therefore, we judged the risk of attrition bias as unclear for the study.
Selective reporting
The protocols were available for seven studies (De Moerloose 2011; Gardin 2007; Lee 2012; Mandelli 2009; Ohtake 2011; Pautas 2010; Récher 2014). Gardin 2007, Ohtake 2011 and Pautas 2010 reported all pre‐planned outcomes and we judged them as low risk for reporting bias. Lee 2012 reported only a few outcomes of the pre‐planned outcomes and we judged the study as high risk of reporting bias. Protocols of De Moerloose 2011, Mandelli 2009 and Récher 2014 did not report pre‐planned outcomes, so we judged the three studies as unclear risk for reporting bias. All other studies whose protocols were not available were judged as unclear risk of reporting bias.
Other potential sources of bias
Baseline characteristics of patients between groups were not balanced for gender in two studies (Masaoka 1996; Pignon 1996), and for platelet count in one study (Vogler 1992). As baseline imbalance can cause bias in the intervention effect estimate (Higgins 2011b), we judged these studies as high risk of other potential sources of bias. No other potential sources of bias were identified for other studies; they were left as unclear risk of bias for this domain.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
See: Table 1; Table 2; Table 3; Table 4.
Comparison 1: Idarubicin (IDA) versus daunorubicin (DNR)
Eighteen studies with 6755 newly diagnosed AML patients evaluated the efficacy and safety of IDA versus DNR in induction therapy.
Primary outcome measures
Overall survival (OS)
Twelve studies with 5976 newly diagnosed AML patients reported on this outcome (Berman 1991; Creutzig 2013; Gardin 2007; Mandelli 1991; Mandelli 2009; Ohtake 2011; Pautas 2010; Récher 2014; Reiffers 1996; Rowe 2004; Vogler 1992; Wiernik 1992). In the main analysis, OS was statistically significantly longer with IDA than with DNR (hazard ratio (HR) 0.90, 95% confidence interval (CI) 0.84 to 0.96, P = 0.0008; I² for heterogeneity = 42%, P = 0.06) (Figure 4). Removing studies where the ratio of total dose of DNR to total dose of IDA was more than 5 (Creutzig 2013; Ohtake 2011; Pautas 2010) reduced the I². However, subgroup analysis did not indicate that there was a relationship between dose ratio and effect size across all the subgroups (see below).
4.
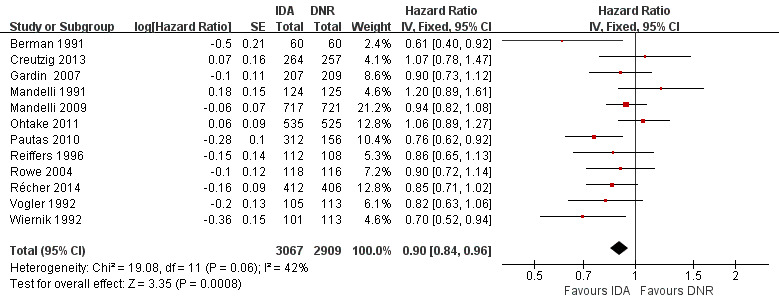
Forest plot of comparison: 1 IDA versus DNR: 1.1 OS‐overall analysis.
Sensitivity analysis
OS remained statistically significantly longer with IDA when we performed sensitivity analyses using random‐effects model (HR 0.89, 95% CI 0.81 to 0.97, P = 0.009; I² for heterogeneity = 43%, P = 0.05) or by excluding one study (Vogler 1992) with high risk of bias (HR 0.90, 95% CI 0.85 to 0.96, P = 0.002; I² for heterogeneity = 46%, P = 0.05).
Subgroup analysis
In the subgroup analysis of OS by dose of IDA (8 mg/m²/d, 9 mg/m²/d, 10 mg/m²/d, 12 mg/m²/d, or 13 mg/m²/d), we did not find sufficient evidence that OS benefit of IDA versus DNR in induction therapy of patients with newly diagnosed AML was different between the subgroups. When the dose of IDA was 8 mg/m²/d, the HR was 0.85 (95% CI 0.74 to 0.99, P = 0.04; two studies, 1038 patients; I² for heterogeneity = 0%, P = 0.95) (Reiffers 1996; Récher 2014). When the dose of IDA was 9 mg/m²/d, the HR was 0.90 (95% CI 0.73 to 1.12, P = 0.36; one study, 416 patients) (Gardin 2007). When the dose of IDA was 10 mg/m²/d, the HR was 0.94 (95% CI 0.82 to 1.08, P = 0.39; one study, 1438 patients) (Mandelli 2009). When the total dose of IDA was 12 mg/m²/d, the HR was 0.92 (95% CI 0.84 to 1.00, P = 0.06; seven studies, 2870 patients; I² for heterogeneity = 60%, P = 0.02) (Berman 1991; Creutzig 2013; Mandelli 1991; Ohtake 2011; Pautas 2010; Rowe 2004; Vogler 1992). When the dose of IDA was 13 mg/m²/d, the HR was 0.70 (95% CI 0.52 to 0.94, P = 0.02; one study, 214 patients) (Wiernik 1992) (P for subgroup differences = 0.42).
In the subgroup analysis according to total dose of DNR (< 180 mg/m² or ≥ 180 mg/m²), we did not find sufficient evidence that OS benefit of IDA versus DNR in induction therapy of patients with newly diagnosed AML was different between the subgroups. When the total dose of DNR was less than 180 mg/m², the HR was 0.89 ( 95% CI 0.82 to 0.97, P = 0.006; eight studies, 3109 patients; I² for heterogeneity = 37%, P = 0.14) (Berman 1991; Gardin 2007; Mandelli 1991; Mandelli 2009; Reiffers 1996; Rowe 2004; Vogler 1992; Wiernik 1992). When the total dose of DNR was more than 180 mg/m², the HR was 0.91 (95% CI 0.82 to 1.00, P = 0.06; four studies, 2867 patients; I² for heterogeneity = 62%, P = 0.05) (Creutzig 2013; Ohtake 2011; Pautas 2010; Récher 2014) (P for subgroup differences = 0.79).
In the subgroup analysis of OS by dose of IDA versus dose of DNR, we did not find sufficient evidence that OS benefit of IDA versus DNR in induction therapy of patients with newly diagnosed AML was different between the subgroups. In the subgroup of 9 mg/m²/d IDA versus 45 mg/m²/d DNR, the HR was 0.90 (95% CI 0.73 to 1.12, P = 0.36; one study, 416 patients) (Gardin 2007). In the subgroup of 12 mg/m²/d IDA versus 45 mg/m²/d DNR, the HR was 0.94 (95% CI 0.81 to 1.09, P = 0.41; three studies, 701 patients; I² for heterogeneity = 48%, P = 0.15) (Mandelli 1991; Rowe 2004; Vogler 1992). In the subgroup of 13 mg/m²/d IDA versus 45 mg/m²/d DNR, the HR was 0.70 (95% CI 0.52 to 0.94, P = 0.02; one study, 214 patients) (Wiernik 1992). In the subgroup of 8 mg/m²/d IDA versus 50 mg/m²/d DNR, the HR was 0.86 (95% CI 0.65 to 1.13, P = 0.28; one study, 220 patients) (Reiffers 1996). In the subgroup of 10 mg/m²/d IDA versus 50 mg/m²/d DNR, the HR was 0.94 (95% CI 0.82 to 1.08, P = 0.39; one study, 1438 patients) (Mandelli 2009). In the subgroup of 12 mg/m²/d IDA versus 50 mg/m²/d DNR, the HR was 0.97 (95% CI 0.83 to 1.14, P = 0.75; two studies, 1180 patients; I² for heterogeneity = 83%, P = 0.01) (Berman 1991; Ohtake 2011). In the subgroup of 8 mg/m²/d IDA versus 60 mg/m²/d DNR, the HR was 0.85 (95% CI 0.71 to 1.02, P = 0.08; one study, 818 patients) (Récher 2014). In the subgroup of 12 mg/m²/d IDA versus 80 mg/m²/d DNR, the HR was 0.83 (95% CI 0.71 to 0.98, P = 0.03; two studies, 989 patients; I² for heterogeneity = 71%, P = 0.06) (Creutzig 2013; Pautas 2010) (P for subgroup differences = 0.56).
In the subgroup analysis of OS by age (< 15 years or ≥ 15 years to < 60 years or ≥60 years), we did not find sufficient evidence that OS benefit of IDA versus DNR in induction therapy of patients with newly diagnosed AML was different between the subgroups. When patients were younger than 15 years, the HR was 1.07 (95% CI 0.78 to 1.47, P = 0.66; one study, 521 patients) (Creutzig 2013). When patients were aged between 15 to 60 years, the HR was 0.88 (95% CI 0.80 to 0.98, P = 0.02; three studies, 2376 patients; I² for heterogeneity = 52%, P = 0.12) (Berman 1991; Mandelli 2009; Récher 2014). When patients were older than 60 years, the HR was 0.95 (95% CI 0.79 to 1.15, P = 0.60; two studies, 527 patients; I² for heterogeneity = 0%, P = 0.36) (Gardin 2007; Vogler 1992) (P for subgroup differences = 0.46).
In the subgroup analysis of OS by cytogenetic risk stratification, we did not find sufficient evidence that OS benefit of IDA versus DNR in induction therapy of patients with newly diagnosed AML was different between the subgroups. In patients with favourable‐risk cytogenetics, the HR was 0.51 (95% CI 0.23 to 1.12, P = 0.09; one study, 123 patients) (Récher 2014). In patients with intermediate‐risk cytogenetics, the HR was 0.70 (95% CI 0.54 to 0.90, P = 0.006; one study, 468 patients) (Récher 2014). In patients with adverse‐risk cytogenetics, the HR was 0.94 (95% CI 0.67 to 1.31, P = 0.72; one study, 174 patients) (Récher 2014) (P for subgroup differences = 0.22).
We did not perform subgroup analysis of OS by AML subtype because the study authors did not provide these subgroup data.
Publication bias
Because there were more than 10 studies included in the meta‐analysis of OS, we assessed the possibility of publication bias with a funnel plot. The obtained funnel plot was symmetrical, which implied low risk of publication bias (Figure 5).
5.
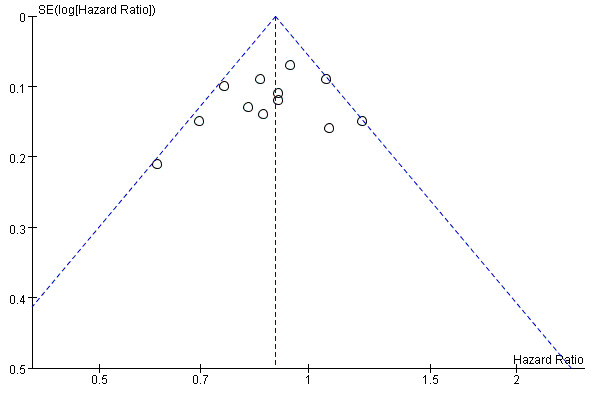
Funnel plot of comparison: 1 IDA versus DNR, outcome: 1.1 OS‐overall analysis.
Disease‐free survival (DFS)
Eight studies including 3070 newly diagnosed AML patients provided data for DFS (Mandelli 1991; Mandelli 2009; Ohtake 2011; Récher 2014; Reiffers 1996; Rowe 2004; Vogler 1992; Wiernik 1992). The main analysis showed a statistically significantly improved DFS for patients receiving IDA in induction therapy compared with those receiving DNR in induction therapy (HR 0.88, 95% CI 0.81 to 0.96, P = 0.004; I² for heterogeneity = 8%, P = 0.36) (Figure 6).
6.
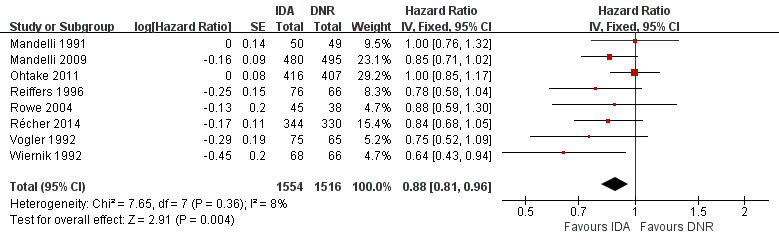
Forest plot of comparison: 1 IDA versus DNR, outcome: 1.9 DFS‐overall analysis.
Sensitivity analysis
The results did not change when we performed sensitivity analysis by random‐effects model (HR 0.88, 95% CI 0.80 to 0.96, P = 0.005; I² for heterogeneity = 8%, P = 0.36).
We did not perform sensitivity analysis based on methodological quality of the studies (including versus excluding studies with high risk of bias) for DFS because all included studies were judged as high risk of bias for blinding regarding all outcomes except OS.
Subgroup analysis
In the subgroup analysis of DFS by dose of IDA (8 mg/m²/d, 10 mg/m²/d, 12 mg/m²/d, or 13 mg/m²/d), we did not find sufficient evidence that DFS benefit of IDA versus DNR in induction therapy of patients with newly diagnosed AML was different between the subgroups. When the dose of IDA was 8 mg/m²/d, the HR was 0.82 (95% CI 0.69 to 0.98, P = 0.03; two studies, 816 patients; I² for heterogeneity = 0%, P = 0.67) (Reiffers 1996; Récher 2014). when the dose of IDA was 10 mg/m²/d, the HR was 0.85 (95% CI 0.71 to 1.02, P = 0.08; one study, 975 patients) (Mandelli 2009). When the dose of IDA was 12 mg/m²/d, the HR was 0.96 (95% CI 0.85 to 1.08, P = 0.48; four studies, 1145 patients; I² for heterogeneity = 0%, P = 0.52) (Mandelli 1991; Ohtake 2011; Rowe 2004; Vogler 1992). When the dose of IDA was 13 mg/m²/d, the HR was 0.64 (95% CI 0.43 to 0.94, P = 0.02; one study, 134 patients) (Wiernik 1992) (P for subgroup differences = 0.16).
In the subgroup analysis according to total dose of DNR (< 180 mg/m² or ≥ 180 mg/m²), we did not find sufficient evidence that DFS benefit of IDA versus DNR in induction therapy of patients with newly diagnosed AML was different between the subgroups. When the total dose of DNR was less than 180 mg/m², the HR was 0.84, (95% CI 0.75 to 0.94, P = 0.002; six studies, 1573 patients; I² for heterogeneity = 0%, P = 0.53) (Mandelli 1991; Mandelli 2009; Reiffers 1996; Rowe 2004; Vogler 1992; Wiernik 1992). When the total dose of DNR was more than 180 mg/m², the HR was 0.94 (95% CI 0.83 to 1.07, P = 0.36; two studies, 1497 patients; I² for heterogeneity = 36%, P = 0.21) (Ohtake 2011; Récher 2014) (P for subgroup differences = 0.16).
In the subgroup analysis of DFS by dose of IDA versus dose of DNR, we did not find sufficient evidence that DFS benefit of IDA versus DNR in induction therapy of patients with newly diagnosed AML was different between the subgroups. In the subgroup of 12 mg/m²/d IDA versus 45 mg/m²/d DNR, the HR was 0.90 (95% CI 0.74 to 1.09, P = 0.27; three studies, 322 patients; I² for heterogeneity = 0%, P = 0.47) (Mandelli 1991; Rowe 2004; Vogler 1992). In the subgroup of 13 mg/m²/d IDA versus 45 mg/m²/d DNR, the HR was 0.64 (95% CI 0.43 to 0.94, P = 0.02; one study, 134 patients) (Wiernik 1992). In the subgroup of 8 mg/m²/d IDA versus 50 mg/m²/d DNR, the HR was 0.78 (95% CI 0.58 to 1.04, P = 0.10; one study, 142 patients) (Reiffers 1996). In the subgroup of 10 mg/m²/d IDA versus 50 mg/m²/d DNR, the HR was 0.85 (95% CI 0.71 to 1.02, P = 0.08; one study, 975 patients) (Mandelli 2009). In the subgroup of 12 mg/m²/d IDA versus 50 mg/m²/d DNR, the HR was 1.00 (95% CI 0.85 to 1.17, P = 1.00; one study, 823 patients) (Ohtake 2011). In the subgroup of 8 mg/m²/d IDA versus 60 mg/m²/d DNR, the HR was 0.84 (95% CI 0.68 to 1.05, P = 0.12; one study, 674 patients) (Récher 2014) (P for subgroup differences = 0.29).
In the subgroup analysis of DFS by cytogenetic risk stratification, we did not find sufficient evidence that DFS benefit of IDA versus DNR in induction therapy of patients with newly diagnosed AML was different between the subgroups. In patients with favourable‐risk cytogenetics, the HR was 0.88 (95% CI 0.47 to 1.64, P = 0.68; one study, 118 patients) (Récher 2014). In patients with intermediate‐risk cytogenetics, the HR was 0.73 (95% CI 0.56 to 0.97, P = 0.03; one study, 393 patients) (Récher 2014). In patients with adverse‐risk cytogenetics, the HR was 0.93 (95% CI 0.62 to 1.41, P = 0.74; one study, 113 patients) (Récher 2014) (P for subgroup differences = 0.61).
No data were available for subgroup analyses of DFS by age and AML subtype.
Secondary outcome measures
Complete remission (CR)
Eighteen studies with 6692 newly diagnosed AML patients reported data on CR (Berman 1991; Creutzig 2001; Creutzig 2013; Eridani 1989; Feng 2010; Gardin 2007; Jia 2011; Lee 2012; Mandelli 1991; Mandelli 2009; Masaoka 1996; Ohtake 2011; Pautas 2010; Récher 2014; Reiffers 1996; Rowe 2004; Vogler 1992; Wiernik 1992). In the main analysis, patients treated with IDA in induction therapy had a statistically significantly improved CR rate compared with those treated with DNR in induction therapy (risk ratio (RR) 1.04, 95% CI 1.01 to 1.07, P = 0.009; I² for heterogeneity = 46%, P = 0.02). Removing studies where the total dose of DNR was less than 180 mg/m² (Berman 1991; Creutzig 2001; Eridani 1989; Feng 2010; Gardin 2007; Jia 2011; Mandelli 1991; Mandelli 2009; Masaoka 1996; Reiffers 1996; Rowe 2004; Vogler 1992; Wiernik 1992) reduced the I² to 13%. However, subgroup analysis did not indicate that there was a relationship between total dose of DNR and effect size across all the subgroups (see below).
Sensitivity analysis
A similar result was obtained when we performed sensitivity analysis using random‐effects model (RR 1.05, 95% CI 1.01 to 1.10, P = 0.03; I² for heterogeneity = 45%, P = 0.02).
We did not perform sensitivity analysis based on methodological quality of the studies for CR rate because all included studies were judged as high risk of bias for blinding regarding all outcomes except OS.
Subgroup analysis
In the subgroup analysis of CR rate by dose of IDA (8 mg/m²/d, 9 mg/m²/d, 10 mg/m²/d, 12 mg/m²/d, or 13 mg/m²/d), we did not find sufficient evidence that the statistically significantly improved CR rate of IDA was different between the subgroups. When the dose of IDA was 8 mg/m²/d, the RR was 1.03 (95% CI 0.97 to 1.09, P = 0.37; three studies, 1083 patients; I² for heterogeneity = 0%, P = 0.58) (Jia 2011; Reiffers 1996; Récher 2014). When the dose of IDA was 9 mg/m²/d, the RR was 1.09 (95% CI 0.92 to 1.29, P = 0.32; one study, 416 patients) (Gardin 2007). When the dose of IDA was 10 mg/m²/d, the RR was 0.99 (95% CI 0.92 to 1.06, P = 0.69; two studies, 1462 patients; I² for heterogeneity = 71%, P = 0.06) (Eridani 1989; Mandelli 2009). When the dose of IDA was 12 mg/m²/d, the RR was 1.05 (95% CI 1.01 to 1.09, P = 0.02; 10 studies, 3446 patients; I² for heterogeneity = 49%, P = 0.04) (Berman 1991; Creutzig 2001; Creutzig 2013; Lee 2012; Mandelli 1991; Masaoka 1996; Ohtake 2011; Pautas 2010; Rowe 2004; Vogler 1992). When the dose of IDA was 13 mg/m²/d, the RR was 1.15 (95% CI 0.94 to 1.42, P = 0.18; one study, 214 patients) (Wiernik 1992) (P for subgroup differences = 0.46).
In the subgroup analysis of CR rate according to total dose of DNR (< 180 mg/m² or ≥ 180 mg/m²), we did not find sufficient evidence that the statistically significantly improved CR rate of IDA was different between the subgroups. When the total dose of DNR was less than 180 mg/m², the RR was 1.06 (95% CI 1.01 to 1.11, P = 0.02; 13 studies, 3671 patients; I² for heterogeneity = 52%, P = 0.01) (Berman 1991; Creutzig 2001; Eridani 1989; Feng 2010; Gardin 2007; Jia 2011; Mandelli 1991; Mandelli 2009; Masaoka 1996; Reiffers 1996; Rowe 2004; Vogler 1992; Wiernik 1992). When the total dose of DNR was more than 180 mg/m², the RR was 1.02 (95% CI 0.98 to 1.06, P = 0.27; five studies, 3021 patients; I² for heterogeneity = 13%, P = 0.33) (Creutzig 2013; Lee 2012; Ohtake 2011; Pautas 2010; Récher 2014) (P for subgroup differences = 0.19).
In the subgroup analysis of CR rate by dose of IDA versus dose of DNR, we did not find sufficient evidence that the statistically significantly improved CR rate of IDA was different between the subgroups. In the subgroup of 8 mg/m²/d IDA versus 25 mg/m²/d DNR, the RR was 1.14 (95% CI 0.64 to 2.03, P = 0.66; one study, 45 patients) (Jia 2011). In the subgroup of 12 mg/m²/d IDA versus 40 mg/m²/d DNR, the RR was 1.46 (95% CI 0.88 to 2.43, P = 0.14; one study, 64 patients) (Masaoka 1996). In the subgroup of 9 mg/m²/d IDA versus 45 mg/m²/d DNR, the RR was 1.09 (95% CI 0.92 to 1.29, P = 0.32; one study, 416 patients) (Gardin 2007). In the subgroup of 10 mg/m²/d IDA versus 45 mg/m²/d DNR, the RR was 3.38 (95% CI 0.90 to 12.74, P = 0.07; one study, 24 patients) (Eridani 1989). In the subgroup of 12 mg/m²/d IDA versus 45 mg/m²/d DNR, the RR was 1.12 (95% CI 0.97 to 1.31, P = 0.13; three studies, 701 patients; I² for heterogeneity = 0%, P = 0.50) (Mandelli 1991; Rowe 2004; Vogler 1992). In the subgroup of 13 mg/m²/d IDA versus 45 mg/m²/d DNR, the RR was 1.15 (95% CI 0.94 to 1.42, P = 0.18; one study, 214 patients) (Wiernik 1992). In the subgroup of 8 mg/m²/d IDA versus 50 mg/m²/d DNR, the RR was 1.11 (95% CI 0.91 to 1.35, P = 0.30; one study, 220 patients) (Reiffers 1996). In the subgroup of 10 mg/m²/d IDA versus 50 mg/m²/d DNR, the RR was 0.98 (95% CI 0.91 to 1.05, P = 0.49; one study, 1438 patients) (Mandelli 2009). In the subgroup of 12 mg/m²/d IDA versus 50 mg/m²/d DNR, the RR was 1.04 (95% CI 0.97 to 1.10, P = 0.25; two studies, 1177 patients; I² for heterogeneity = 82%, P = 0.02) (Berman 1991; Ohtake 2011). In the subgroup of 8 mg/m²/d IDA versus 60 mg/m²/d DNR, the RR was 1.01 (95% CI 0.95 to 1.07, P = 0.78; one study, 818 patients) (Récher 2014). In the subgroup of 12 mg/m²/d IDA versus 60 mg/m²/d DNR, the RR was 0.99 (95% CI 0.91 to 1.07, P = 0.78; one study, 358 patients) (Creutzig 2001). In the subgroup of 12 mg/m²/d IDA versus 80 mg/m²/d DNR, the RR was 1.05 (95% CI 0.99 to 1.11, P = 0.13; two studies, 989 patients; I² for heterogeneity = 80%, P = 0.03) (Creutzig 2013; Pautas 2010). In the subgroup of 12 mg/m²/d IDA versus 90 mg/m²/d DNR, the RR was 0.99 (95% CI 0.84 to 1.16, P = 0.86; one study, 157 patients) (Lee 2012) (P for subgroup differences = 0.39).
In the subgroup analysis by age, we did not find sufficient evidence that the statistically significantly improved CR rate of IDA was different between the subgroups. In patients younger than 15 years, the RR was 0.99 (95% CI 0.93 to 1.05, P = 0.77; one study, 521 patients) (Creutzig 2013). In patients aged between 15 and 60 years, the RR was 1.04 (95% CI 1.00 to 1.08, P = 0.05; eight studies, 3294 patients; I² for heterogeneity = 63%, P = 0.009) (Berman 1991; Eridani 1989; Mandelli 2009; Masaoka 1996; Ohtake 2011; Récher 2014; Vogler 1992; Wiernik 1992). In patients older than 60 years, the RR was 1.08 (95% CI 0.95 to 1.22, P = 0.25; six studies, 751 patients; I² for heterogeneity = 0%, P = 0.77) (Eridani 1989; Gardin 2007; Masaoka 1996; Reiffers 1996; Vogler 1992; Wiernik 1992) (P for subgroup differences = 0.32).
Subgroup analysis by cytogenetic risk stratification indicated that CR benefit of IDA versus DNR in induction therapy of patients with newly diagnosed AML was different between the subgroups. In patients with favourable‐risk cytogenetics, the RR was 0.95 (95% CI 0.88 to 1.01, P = 0.10; two studies, 280 patients; I² for heterogeneity = 0%, P = 0.95) (Ohtake 2011; Pautas 2010). In patients with intermediate‐risk cytogenetics, the RR was 1.04 (95% CI 0.98 to 1.11, P = 0.18; three studies, 1080 patients; I² for heterogeneity = 37%, P = 0.20) (Creutzig 2001; Ohtake 2011; Pautas 2010). In patients with adverse‐risk cytogenetics, the RR was 1.12 (95% CI 0.97 to 1.28, P = 0.12; three studies, 359 patients; I² for heterogeneity = 4%, P = 0.35) (Creutzig 2001; Ohtake 2011; Pautas 2010) (P for subgroup differences = 0.03).
None of the studies provided data for subgroup analysis of CR by AML subtype.
Publication bias
We assessed the possibility of publication bias for CR with a funnel plot. The plot showed an imbalance of positive and negative results for IDA, indicating that studies with negative results for IDA might remain unpublished. Taking this into consideration, the estimated benefit of IDA versus DNR in improving CR rate may be overestimated.
Death on induction therapy
Fourteen studies with 6349 newly diagnosed AML patients assessed death on induction therapy (Berman 1991; Creutzig 2001; Creutzig 2013; Eridani 1989; Gardin 2007; Mandelli 1991; Mandelli 2009; Ohtake 2011; Pautas 2010; Récher 2014; Reiffers 1996; Rowe 2004; Vogler 1992; Wiernik 1992). The main analysis showed a statistically significantly increased risk of death on induction therapy for patients receiving IDA compared with those receiving DNR (RR 1.18, 95% CI 1.01 to 1.36, P = 0.03; I² for heterogeneity = 25%, P = 0.19).
Sensitivity analysis
Sensitivity analysis using random‐effects model showed that the risk of death on induction therapy was similar between groups (RR 1.17, 95% CI 0.97 to 1.41, P = 0.11; I² for heterogeneity = 25%, P = 0.19).
We did not perform sensitivity analysis based on methodological quality of the studies for the risk of death on induction therapy because all included studies were judged as high risk of bias for blinding regarding all outcomes except OS.
Subgroup analysis
In the subgroup analysis by dose of IDA (8 mg/m²/d, 9 mg/m²/d, 10 mg/m²/d, 12 mg/m²/d, or 13 mg/m²/d), we did not find sufficient evidence that the statistically significantly increased risk of death on induction therapy of IDA was different between the subgroups. When the dose of IDA was 8 mg/m²/d, the RR was 1.20 (95% CI 0.79 to 1.83, P = 0.40; two studies, 1038 patients; I² for heterogeneity = 0%, P = 0.50) (Reiffers 1996; Récher 2014). When the dose of IDA was 9 mg/m²/d, the RR was 0.91 (95% CI 0.51 to 1.65, P = 0.76; one study, 416 patients) (Gardin 2007). When the dose of IDA was 10 mg/m²/d, the RR was 1.09 (95% CI 0.83 to 1.41, P = 0.54; two studies, 1462 patients; I² for heterogeneity = 62%, P = 0.10) (Eridani 1989; Mandelli 2009). When the dose of IDA was 12 mg/m²/d, the RR was 1.30 (95% CI 1.04 to 1.63, P = 0.02; eight studies, 3225 patients; I² for heterogeneity = 44%, P = 0.09) (Berman 1991; Creutzig 2001; Creutzig 2013; Mandelli 1991; Ohtake 2011; Pautas 2010; Rowe 2004; Vogler 1992). When the dose of IDA was 13 mg/m²/d, the RR was 1.14 (95% CI 0.67 to 1.96, P = 0.62; one study, 208 patients) (Wiernik 1992) (P for subgroup differences = 0.77).
In the subgroup analysis according to total dose of DNR (< 180 mg/m² or ≥ 180 mg/m²), we did not find sufficient evidence that the statistically significantly increased risk of death on induction therapy of IDA was different between the subgroups. When the total dose of DNR was less than 180 mg/m², the RR was 1.17 (95% CI 1.00 to 1.38, P = 0.05; 10 studies, 3485 patients; I² for heterogeneity = 14%, P = 0.32) (Berman 1991; Creutzig 2001; Eridani 1989; Gardin 2007; Mandelli 1991; Mandelli 2009; Reiffers 1996; Rowe 2004; Vogler 1992; Wiernik 1992). When the total dose of DNR was more than 180 mg/m², the RR was 1.19 (95% CI 0.83 to 1.70, P = 0.35; 4 studies, 2864 patients; I² for heterogeneity = 57%, P = 0.07) (Creutzig 2013; Ohtake 2011; Pautas 2010; Récher 2014) (P for subgroup differences = 0.95).
In the subgroup analysis by dose of IDA versus dose of DNR, we did not find sufficient evidence that the statistically significantly increased risk of death on induction therapy of IDA was different between the subgroups. In the subgroup of 9 mg/m²/d IDA versus 45 mg/m²/d DNR, the RR was 0.91 (95% CI 0.51 to 1.65, P = 0.76; one study, 416 patients) (Gardin 2007). In the subgroup of 10 mg/m²/d IDA versus 45 mg/m²/d DNR, the RR was 0.34 (95% CI 0.08 to 1.41, P = 0.14; one study, 24 patients) (Eridani 1989). In the subgroup of 12 mg/m²/d IDA versus 45 mg/m²/d DNR, the RR was 1.29 (95% CI 0.98 to 1.70, P = 0.07; three studies, 701 patients; I² for heterogeneity = 62%, P = 0.07) (Mandelli 1991; Rowe 2004; Vogler 1992). In the subgroup of 13 mg/m²/d IDA versus 45 mg/m²/d DNR, the RR was 1.14 (95% CI 0.67 to 1.96, P = 0.62; one study, 208 patients) (Wiernik 1992). In the subgroup of 8 mg/m²/d IDA versus 50 mg/m²/d DNR, the RR was 1.39 (95% CI 0.78 to 2.48, P = 0.27; one study, 220 patients) (Reiffers 1996). In the subgroup of 10 mg/m²/d IDA versus 50 mg/m²/d DNR, the RR was 1.13 (95% CI 0.87 to 1.48, P = 0.36; one study, 1438 patients) (Mandelli 2009). In the subgroup of 12 mg/m²/d IDA versus 50 mg/m²/d DNR, the RR was 1.91 (95% CI 1.04 to 3.53, P = 0.04; two studies, 1177 patients; I² for heterogeneity = 9%, P = 0.29) (Berman 1991; Ohtake 2011). In the subgroup of 8 mg/m²/d IDA versus 60 mg/m²/d DNR, the RR was 1.04 (95% CI 0.56 to 1.91, P = 0.91; one study, 818 patients) (Récher 2014). In the subgroup of 12 mg/m²/d IDA versus 60 mg/m²/d DNR, the RR was 1.72 (95% CI 0.59 to 5.04, P = 0.32; one study, 358 patients) (Creutzig 2001). In the subgroup of 12 mg/m²/d IDA versus 80 mg/m²/d DNR, the RR was 0.77 (95% CI 0.42 to 1.42, P = 0.40; two studies, 989 patients; I² for heterogeneity = 34%, P = 0.22) (Creutzig 2013; Pautas 2010) (P for subgroup differences = 0.40).
In the subgroup analysis of death on induction therapy by age, we did not find sufficient evidence that the statistically significantly increased risk of death on induction therapy of IDA was different between the subgroups. When patients were younger than 15 years, the RR was 1.36 (95% CI 0.44 to 4.24, P = 0.59; one study, 521 patients) (Creutzig 2013). When patients were aged between 15 and 60 years, the RR was 1.08 (95% CI 0.85 to 1.37, P = 0.55; four studies, 2390 patients; I² for heterogeneity = 0%, P = 0.44) (Berman 1991; Eridani 1989; Mandelli 2009; Récher 2014). When patients were older than 60 years, the RR was 0.92 (95% CI 0.53 to 1.60, P = 0.77; two studies, 426 patients; I² for heterogeneity = 0%, P = 0.91) (Eridani 1989; Gardin 2007) (P for subgroup differences = 0.80).
There were no enough data to analyse death on induction therapy according to cytogenetic risk stratification and AML subtype.
Publication bias
The possibility of publication bias for the outcome of death on induction therapy was assessed with a funnel plot. The obtained funnel plot was symmetrical indicating low risk of publication bias.
Relapse
Four studies with 1091 newly diagnosed AML patients provided information regarding relapse (Creutzig 2001; Pautas 2010; Reiffers 1996; Vogler 1992). The main analysis showed a statistically significantly reduced relapse rate for patients with IDA compared with those with DNR in induction therapy (RR 0.88, 95% CI 0.80 to 0.98, P = 0.02; I² for heterogeneity = 69%, P = 0.02). Removing studies where the total dose of DNR was less than 180 mg/m² (Reiffers 1996; Vogler 1992) or those where the total dose of DNR was more than 180 mg/m² (Creutzig 2013; Pautas 2010) both reduced the I² to 0%. Subgroup analysis indicated that there was a relationship between total dose of DNR and effect size across all the subgroups (see below).
Sensitivity analysis
No statistically significant difference in relapse rate was found between arms when we performed sensitivity analysis by random‐effects model (RR 0.85, 95% CI 0.71 to 1.01, P = 0.06; I² for heterogeneity = 69%, P = 0.02).
We did not perform sensitivity analysis based on methodological quality of the studies for relapse rate because all included studies were judged as high risk of bias for blinding regarding all outcomes except OS.
Subgroup analysis
The subgroup analysis of relapse rate by dose of IDA (8 mg/m²/d or 12 mg/m²/d) indicated that the effect of reducing relapse rate of IDA versus DNR in induction therapy of patients with newly diagnosed AML was different between the subgroups. When the dose of IDA was 8 mg/m²/d, the RR was 0.75 (95% CI 0.66 to 0.86, P < 0.0001; one study, 131 patients) (Reiffers 1996). However, when the dose of IDA was 12 mg/m²/d, the RR was 0.92 (95% CI 0.81 to 1.04, P = 0.18; three studies, 960 patients; I² for heterogeneity = 54%, P = 0.11) (Creutzig 2013; Pautas 2010; Vogler 1992) (P for subgroup differences = 0.03).
The subgroup analysis by total dose of DNR (< 180 mg/m² or ≥ 180 mg/m²) indicated that the effect of reducing relapse rate of IDA versus DNR in induction therapy of patients with newly diagnosed AML was different between the subgroups. When the total dose of DNR was less than 180 mg/m², the RR was 0.74 (95% CI 0.65 to 0.85, P < 0.0001; two studies, 271 patients; I² for heterogeneity = 0%, P = 0.74) (Reiffers 1996; Vogler 1992). However, when the total dose of DNR was more than 180 mg/m², the RR was 0.97, 95% CI 0.84 to 1.13, P = 0.73; two studies, 820 patients; I² for heterogeneity = 0%, P = 0.37) (Creutzig 2013; Pautas 2010) (P for subgroup differences = 0.006).
The subgroup analysis by dose of IDA versus dose of DNR indicated that the effect of reducing relapse rate of IDA versus DNR in induction therapy of patients with newly diagnosed AML was different between the subgroups. In the subgroups of 12 mg/m²/d IDA versus 45 mg/m²/d DNR and 8 mg/m²/d IDA versus 50 mg/m²/d DNR, the RR was 0.72 (95% CI 0.56 to 0.93, P = 0.01; one study, 140 patients) (Vogler 1992) and 0.75 (95% CI 0.66 to 0.86, P < 0.0001; one study, 131 patients) (Reiffers 1996), respectively. However, in the subgroup of 12 mg/m²/d IDA versus 80 mg/m²/d DNR, the RR was 0.97 (95% CI 0.84 to 1.13, P = 0.73; two studies, 820 patients; I² for heterogeneity = 0%, P = 0.37) (Creutzig 2013; Pautas 2010) (P for subgroup differences = 0.02).
None of studies reported data for subgroup analyses of relapse by cytogenetic risk stratification, age and AML subtype.
Adverse events (AEs)
The following grade 3/4 AEs which were reported by more than one study were included in meta‐analyses: nausea/vomiting (Berman 1991; Masaoka 1996; Reiffers 1996; Vogler 1992), alopecia (Masaoka 1996; Reiffers 1996; Vogler 1992; Wiernik 1992), diarrhoea (Masaoka 1996; Reiffers 1996; Vogler 1992), hepatic toxicity (Creutzig 2013; Mandelli 1991; Masaoka 1996; Reiffers 1996; Vogler 1992), renal toxicity (Mandelli 1991; Masaoka 1996; Reiffers 1996; Vogler 1992), cardiac toxicity (Creutzig 2013; Mandelli 1991; Masaoka 1996; Ohtake 2011; Récher 2014; Vogler 1992), skin toxicity (Creutzig 2013; Masaoka 1996; Vogler 1992), cental neurotoxicity (Creutzig 2013; Reiffers 1996), bleeding (Masaoka 1996; Ohtake 2011; Pautas 2010; Récher 2014), stomatitis (Masaoka 1996; Reiffers 1996), mucositis (Berman 1991; Creutzig 2013; Pautas 2010; Récher 2014; Vogler 1992), infection (Creutzig 2001; Creutzig 2013; Mandelli 2009; Masaoka 1996; Récher 2014) and sepsis (Ohtake 2011; Pautas 2010). Further grade 3/4 AEs that were reported by only one study were listed in Table 5.
1. Grade 3/4 adverse events (reported by one study).
| Comparison | Name of study | Grade 3/4 AEs | Experimental arm (N) | Control arm (N) | P value |
| IDA versus DNR | |||||
| Masaoka 1996 | Fever | 7/32 | 7/32 | 1.00 | |
| Tachycardia | 0/32 | 1/32 | 0.50 | ||
| Anorexia | 11/32 | 12/32 | 0.79 | ||
| Pain | 1/32 | 0/31 | 0.51 | ||
| Creutzig 2013 | General condition | 116/260 | 86/223 | 0.18 | |
| Pulmonary toxicity | 35/248 | 33/204 | 0.54 | ||
| Ohtake 2011 | Febrile neutropenia | 416/532 | 406/525 | 0.74 | |
| Mandelli 2009 | AEs other than infection | 330/717 | 321/721 | 0.57 | |
| IDA versus MIT | |||||
| Archimbaud 1999 | Haemorrhage | 5/80 | 4/80 | 0.73 | |
| Cardiac toxicity | 2/80 | 3/80 | 0.65 | ||
| Fever/infection | 33/80 | 30/80 | 0.63 | ||
| Beksac 1998 | Alopecia | 13/34 | 12/29 | 0.80 | |
| Rash | 2/34 | 1/29 | 0.66 | ||
| De Moerloose 2011 | Fever | 28/112 | 26/115 | 0.67 | |
| Mandelli 2009 | AEs other than infection | 330/717 | 330/719 | 0.96 |
AEs: adverse events; DNR: daunorubicin; IDA: idarubicin; MIT: mitoxantrone.
The main analysis of grade 3/4 mucositis showed a statistically significantly increased risk of grade 3/4 mucositis with IDA versus DNR (RR 1.22, 95% CI 1.04 to 1.44, P = 0.02; five studies, 2000 patients; I² for heterogeneity = 42%, P = 0.14) (Berman 1991; Creutzig 2013; Pautas 2010; Récher 2014; Vogler 1992). However, the sensitivity analysis by random‐effects model showed no statistically significant difference in the risk of grade 3/4 mucositis between arms (RR 1.22, 95% CI 0.92 to 1.61, P = 0.17; I² for heterogeneity = 42%, P = 0.14).
Both the main analyses and sensitivity analyses showed no statistically significant differences between arms in the risks of following grade 3/4 AEs:
grade 3/4 nausea/vomiting (four studies, N = 622; main analysis: RR 1.12, 95% CI 0.73 to 1.73, P = 0.60; sensitivity analysis by random‐effects model: RR 1.07, 95% CI 0.69 to 1.65, P = 0.78);
grade 3/4 alopecia (four studies, N = 715; main analysis: RR 1.08, 95% CI 0.90 to 1.31, P = 0.40; sensitivity analysis by random‐effects model: RR 1.08, 95% CI 0.86 to 1.35, P = 0.53);
grade 3/4 diarrhoea (three studies, N = 502; main analysis: RR 1.25, 95% CI 0.68 to 2.28, P = 0.48; sensitivity analysis by random‐effects model: RR 1.25, 95% CI 0.68 to 2.29, P = 0.47);
grade 3/4 hepatic toxicity (five studies, N = 1226; main analysis: RR 0.84, 95% CI 0.59 to 1.20, P = 0.34; sensitivity analysis by random‐effects model: RR 0.93, 95% CI 0.57 to 1.52, P = 0.78);
grade 3/4 renal toxicity (four studies, N = 743; main analysis: RR 1.73, 95% CI 0.75 to 4.00, P = 0.20; sensitivity analysis by random‐effects model: RR 1.68, 95% CI 0.70 to 4.04, P = 0.25);
grade 3/4 cardiac toxicity (six studies, N = 2795; main analysis: RR 0.98, 95% CI 0.70 to 1.37, P = 0.91; sensitivity analysis by random‐effects model: RR 0.98, 95% CI 0.67 to 1.43, P = 0.91);
grade 3/4 skin toxicity (three studies, N = 761; main analysis: RR 1.02, 95% CI 0.47 to 2.23, P = 0.96; sensitivity analysis by random‐effects model: RR 1.02, 95% CI 0.41 to 2.50, P = 0.97);
grade 3/4 central neurotoxicity (two studies, N = 707; main analysis: RR 1.29, 95% CI 0.49 to 3.35, P = 0.61; sensitivity analysis by random‐effects model: RR 1.28, 95% CI 0.49 to 3.35, P = 0.61);
grade 3/4 bleeding (four studies, N = 2299; main analysis: RR 0.97, 95% CI 0.65 to 1.45, P = 0.89; sensitivity analysis by random‐effects model: RR 0.97, 95% CI 0.65 to 1.45, P = 0.87);
grade 3/4 stomatitis (two studies, N = 284; main analysis: RR 1.65, 95% CI 0.78 to 3.47, P = 0.19; sensitivity analysis by random‐effects model: RR 1.59, 95% CI 0.75 to 3.38, P = 0.23);
grade 3/4 infection (five studies, N = 3095; main analysis: RR 1.07, 95% CI 0.99 to 1.14, P = 0.08; sensitivity analysis by random‐effects model: RR 1.04, 95% CI 0.97 to 1.11, P = 0.28);
grade 3/4 sepsis (two studies, N = 1417; main analysis: RR 1.30, 95% CI 0.96 to 1.77, P = 0.09; sensitivity analysis by random‐effects model: RR 1.30, 95% CI 0.74 to 2.28, P = 0.37).
We did not perform sensitivity analysis based on methodological quality of the studies for the above grade 3/4 AEs because all included studies were judged as high risk of bias for blinding regarding all outcomes except OS.
Subgroup analysis
The subgroup analyse of grade 3/4 hepatic toxicity by total dose of DNR indicated that there was a relationship between total dose of DNR and effect size across all the subgroups. When the total dose of DNR was ≧ 180 mg/m², the RR was 0.55 (95% CI 0.32 to 0.93, P = 0.02; one study, 483 patients) (Creutzig 2013). However, when the total dose of DNR was < 180 mg/m², the RR was 1.25 (95% CI 0.75 to 2.07, P = 0.39; four studies, 743 patients; I² for heterogeneity = 0%, P = 0.77) (Mandelli 1991; Masaoka 1996; Reiffers 1996; Vogler 1992) (P for subgroup differences = 0.03).
The subgroup analyses of grade 3/4 nausea/vomiting, alopecia, diarrhoea, hepatic toxicity, renal toxicity, cardiac toxicity, central neurotoxicity, bleeding, mucositis and infection by dose of IDA both showed no interaction between dose of IDA and effect size across all the subgroups (all P for subgroup differences > 0.05).
The subgroup analyses of grade 3/4 cardiac toxicity, skin toxicity, central neurotoxicity, bleeding, mucositis and infection by total dose of DNR both showed no interaction between total dose of DNR and effect size across all the subgroups (all P for subgroup differences > 0.05).
The subgroup analyses of grade 3/4 nausea/vomiting, alopecia, diarrhoea, hepatic toxicity, renal toxicity, cardiac toxicity, skin toxicity, central neurotoxicity, bleeding, stomatitis, mucositis, infection and sepsis by dose of IDA versus dose of DNR showed no interaction between dose ratio and effect size across all the subgroups (all P for subgroup differences > 0.05).
The subgroup analyses of grade 3/4 mucositis and infection by age showed no interaction between age and effect size across all the subgroups (all P for subgroup differences > 0.05).
We did not perform subgroup analyses for other grade 3/4 AEs and neither did we perform subgroup analyses by cytogenetic risk stratification or AML subtype as no subgroup data were available.
Quality of life (QoL)
None of the studies reported data regarding QoL.
Comparison 2: IDA versus MIT
Eight studies including 2419 newly diagnosed AML patients assessed the role of IDA versus MIT in induction therapy.
Primary outcome measures
Overall survival (OS)
Six studies with 2171 newly diagnosed AML patients reported OS data (Archimbaud 1999; Beksac 1998; De Moerloose 2011; Indrak 2001; Mandelli 2009; Rowe 2004). The main analysis showed no statistically significant difference in OS between patients with IDA and those with MIT (HR 0.98, 95% CI 0.89 to 1.08, P = 0.69; I² for heterogeneity = 38%, P = 0.15). As shown in the sensitivity analysis, the heterogeneity was mainly explained by the study with high risk of bias (Beksac 1998). By excluding the study heterogeneity was reduced to 0%.
Sensitivity analysis
Similar results were obtained in the sensitivity analyses either by random‐effects model (HR 0.97, 95% CI 0.83 to 1.12, P = 0.65; I² for heterogeneity = 38%, P = 0.15) or by excluding one study (Beksac 1998) with high risk of bias (HR 1.01, 95% CI 0.92 to 1.11, P = 0.87; I² for heterogeneity = 0%, P = 0.98).
Subgroup analysis
The subgroup analysis of OS by dose of IDA (8 mg/m²/d, 10 mg/m²/d or 12 mg/m²/d) showed no interaction between dose of IDA and effect size across all the subgroups. When the dose of IDA was 8 mg/m²/d, the HR was 1.06 (95% CI 0.81 to 1.39, P = 0.66; two studies, 220 patients; I² for heterogeneity = 0%, P = 0.70) (Archimbaud 1999; Indrak 2001). When the dose of IDA was 10 mg/m²/d, the HR was 0.99 (95% CI 0.89 to 1.11, P = 0.91; two studies, 1663 patients; I² for heterogeneity = 0%, P = 0.85) (De Moerloose 2011; Mandelli 2009). When the dose of IDA was 12 mg/m²/d, the HR was 0.90 (95% CI 0.73 to 1.11, P = 0.31; two studies, 288 patients; I² for heterogeneity = 85%, P = 0.009) (Beksac 1998; Rowe 2004) (P for subgroup differences = 0.58).
The subgroup analysis of OS by dose of age showed no interaction between age and effect size across all the subgroups. In patients aged between 15 and 60 years, the HR was 0.99 (95% CI 0.88 to 1.11, P = 0.87; one study, 1436 patients) (Mandelli 2009). In patients older than 60 years, the HR was 1.03 (95% CI 0.75 to 1.14, P = 0.85; one study, 160 patients) (Archimbaud 1999) (P for subgroup differences = 0.81).
No data were available for subgroup analyses of OS according to cytogenetic risk stratification and AML subtype.
Disease‐free survival (DFS)
Four studies including 249 newly diagnosed AML patients provided data for DFS (Archimbaud 1999; Beksac 1998; Indrak 2001; Rowe 2004). There was no statistically significantly difference in DFS between patients treated with IDA or MIT in induction therapy (HR 0.88, 95% CI 0.70 to 1.10, P = 0.26; I² for heterogeneity = 45%, P = 0.14).
Sensitivity analysis
The DFS remained similar between arms when we performed sensitivity analysis by random‐effects model (HR 0.86, 95% CI 0.62 to 1.21, P = 40; I² for heterogeneity = 45%, P = 0.14).
We did not perform sensitivity analysis based on methodological quality of the studies for DFS because all included studies were judged as high risk of bias for blinding regarding all outcomes except OS.
Subgroup analysis
The subgroup analysis of DFS by dose of IDA (8 mg/m²/d or 12 mg/m²/d) showed no interaction between dose of IDA and effect size across all the subgroups. When the dose of IDA was 8 mg/m²/d, the HR was 1.06 (95% CI 0.75 to 1.50, P = 0.73; two studies, 123 patients; I² for heterogeneity = 0%, P = 0.41) (Archimbaud 1999; Indrak 2001). When the dose of IDA was 12 mg/m²/d, the HR was 0.75 (95% CI 0.55 to 1.02, P = 0.07; two studies, 126 patients; I² for heterogeneity = 63%, P = 0.10) (Beksac 1998; Rowe 2004) (P for subgroup differences = 0.14).
None of the studies reported data for subgroup analyses of DFS according to cytogenetic risk stratification, age and AML subtype.
Secondary outcome measures
Complete remission (CR)
Eight studies with 2411 newly diagnosed AML patients reported on CR (Archimbaud 1999; Beksac 1998; De Moerloose 2011; Feng 2010; Indrak 2001; Mandelli 2009; Rowe 2004; Wang 2011). The main analysis of CR showed no statistically significantly difference between arms (RR 0.97, 95% CI 0.92 to 1.03, P = 0.32; I² for heterogeneity = 0%, P = 0.50).
Sensitivity analysis
Similar results were obtained when we performed sensitivity analysis by random‐effects model (RR 0.97, 95% CI 0.92 to 1.02, P = 0.25; I² for heterogeneity = 0%, P = 0.50).
We did not perform sensitivity analysis based on methodological quality of the studies for CR rate because all included studies were judged as high risk of bias for blinding regarding all outcomes except OS.
Subgroup analysis
The subgroup analysis by dose of IDA (8 mg/m²/d, 10 mg/m²/d or 12 mg/m²/d) showed no interaction between dose of IDA and effect size across all the subgroups. When the dose of IDA was 8 mg/m²/d, the RR was 0.88 (95% CI 0.70 to 1.11, P = 0.28; two studies, 220 patients; I² for heterogeneity = 0%, P = 0.73) (Archimbaud 1999; Indrak 2001). When the dose of IDA was 10 mg/m²/d, the RR was 0.95 (95% CI 0.90 to 1.02, P = 0.14; two studies, 1663 patients; I² for heterogeneity = 0%, P = 0.69) (De Moerloose 2011; Mandelli 2009). When the dose of IDA was 12 mg/m²/d, the RR was 0.99 (95% CI 0.80 to 1.24, P = 0.96; two studies, 295 patients;I² for heterogeneity = 0%, P = 0.44) (Beksac 1998; Rowe 2004) (P for subgroup differences = 0.74).
The subgroup analysis of CR according to age showed no interaction between age and effect size across all the subgroups. In patients aged between 15 and 60 years, the RR was 0.96 (95% CI 0.90 to 1.03, P = 0.23; two studies, 1490 patients; I² for heterogeneity = 0%, P = 0.98) (Beksac 1998; Mandelli 2009). In patients older than 60 years, the RR was 0.91 (95% CI 0.71 to 1.18, P = 0.49; two studies, 169 patients; I² for heterogeneity = 0%, P = 0.48) (Archimbaud 1999; Beksac 1998) (P for subgroup differences = 0.73).
Data were not available for subgroup analyses of CR by cytogenetic risk stratification and AML subtype.
Death on induction therapy
Five studies including 2055 newly diagnosed AML patients analysed death on induction therapy (Archimbaud 1999; Beksac 1998; Mandelli 2009; Rowe 2004; Wang 2011). No statistically significant difference in the risk of death on induction therapy was found between patients with IDA and those with MIT in induction therapy (RR 1.10, 95% CI 0.88 to 1.38, P = 0.39; I² for heterogeneity = 0%, P = 0.68).
Sensitivity analysis
The result did not change when we performed sensitivity analysis by random‐effects model (RR 1.10, 95% CI 0.88 to 1.38, P = 0.38; I² for heterogeneity = 0%, P = 0.68).
We did not perform sensitivity analysis based on methodological quality of the studies for the risk of death on induction therapy because all included studies were judged as high risk of bias for blinding regarding all outcomes except OS.
Subgroup analysis
The subgroup analysis by dose of IDA (8 mg/m²/d, 10 mg/m²/d or 12 mg/m²/d) showed no interaction between dose of IDA and effect size across all the subgroups. When the dose of IDA was 8 mg/m²/d, the RR was 0.80 (95% CI 0.33 to 1.92, P = 0.62; one study, 160 patients) (Archimbaud 1999). When the dose of IDA was 10 mg/m²/d, the RR was 1.06 (95% CI 0.81 to 1.38, P = 0.68; one study, 1436 patients) (Mandelli 2009). When the dose of IDA was 12 mg/m²/d, the RR was 1.48 (95% CI 0.90 to 2.43, P = 0.12; two studies, 295 patients; I² for heterogeneity = 0%, P = 0.65) (Beksac 1998; Rowe 2004) (P for subgroup differences = 0.38).
The subgroup analysis of death on induction therapy by age showed no interaction between age and effect size across all the subgroups. In patients aged between 15 and 60 years, the RR was 1.06 (95% CI 0.81 to 1.38, P = 0.68; one study, 1436 patients) (Mandelli 2009). In patients older than 60 years, the RR was 0.80 (95% CI 0.33 to 1.92, P = 0.62; one study, 160 patients) (Archimbaud 1999) (P for subgroup differences = 0.55).
There were no data for subgroup analyses of death on induction therapy by cytogenetic risk stratification and AML subtype.
Relapse
Data of relapse were reported by three studies with 328 newly diagnosed AML patients (Archimbaud 1999; Beksac 1998; De Moerloose 2011). In the main analysis, the relapse rate was similar between patients treated with IDA and those treated with MIT in induction therapy (RR 0.99, 95% CI 0.80 to 1.22, P = 0.89; I² for heterogeneity = 0%, P = 0.80).
Sensitivity analysis
The relapse rate remained similar between the two groups when we performed sensitivity analysis by random‐effects model (RR 0.97, 95% CI 0.80 to 1.19, P = 0.80; I² for heterogeneity = 0%, P = 0.80).
We did not perform sensitivity analysis based on methodological quality of the studies for relapse rate because all included studies were judged as high risk of bias for blinding regarding all outcomes except OS.
Subgroup analysis
In the subgroup analysis by dose of IDA (8 mg/m²/d, 10 mg/m²/d or 12 mg/m²/d) showed no interaction between dose of IDA and effect size across all the subgroups. When the dose of IDA was 8 mg/m²/d, the RR was 1.07 (95% CI 0.75 to 1.53, P = 0.71; one study, 95 patients) (Archimbaud 1999). When the dose of IDA was 10 mg/m²/d, the RR was 0.96 (95% CI 0.68 to 1.37, P = 0.84; one study, 187 patients) (De Moerloose 2011). When the dose of IDA was 12 mg/m²/d, the RR was 0.91 (95% CI 0.66 to 1.26, P = 0.58; one study, 46 patients) (Beksac 1998) (P for subgroup differences = 0.81).
No data were available for subgroup analyses of relapse rate by age, cytogenetic risk stratification and AML subtype.
Adverse events (AEs)
We included the following grade 3/4 AEs in meta‐analyses because they were reported by more than one study: nausea/vomiting (Archimbaud 1999; Beksac 1998; Wang 2011), diarrhoea (Archimbaud 1999; Beksac 1998), hepatic toxicity (Archimbaud 1999; Wang 2011), renal toxicity (Archimbaud 1999; Beksac 1998), mucositis (Archimbaud 1999; Beksac 1998), and infection (De Moerloose 2011; Mandelli 2009). One study (Archimbaud 1999) showed that there was no statistically significant difference in the risk of grade 3/4 cardiac toxicity between arms (one study, N = 160; RR 0.67, 95% CI 0.11 to 3.88, P = 0.65), and other grade 3/4 AEs reported by only one study are listed in Table 5.
No statistically significant differences between patients with IDA‐based induction therapy and those with MIT‐based induction therapy were found both in the main analyses and in the sensitivity analyses for the following grade 3/4 AEs:
grade 3/4 nausea/vomiting (three studies, N = 387; main analysis: RR 1.03, 95% CI 0.66 to 1.61, P = 0.90; sensitivity analysis by random‐effects model: RR 1.04, 95% CI 0.67 to 1.63, P = 0.86);
grade 3/4 diarrhoea (two studies, N = 223; main analysis: RR 1.41, 95% CI 0.68 to 2.89, P = 0.36; sensitivity analysis by random‐effects model: RR 1.40, 95% CI 0.68 to 2.88, P = 036);
grade 3/4 hepatic toxicity (two studies, N = 324; main analysis: RR 1.22, 95% CI 0.47 to 3.17, P = 0.69; sensitivity analysis by random‐effects model: RR 1.21, 95% CI 0.47 to 3.16, P = 0.69);
grade 3/4 renal toxicity (two studies, N = 223; main analysis: RR 0.31, 95% CI 0.03 to 2.91, P = 0.30; sensitivity analysis by random‐effects model: RR 0.31, 95% CI 0.03 to 2.91, P = 0.30);
grade 3/4 mucositis (two studies, N = 223; main analysis: RR 1.89, 95% CI 0.36 to 9.92, P = 0.45; sensitivity analysis by random‐effects model: RR 1.81, 95% CI 0.32 to 10.21, P = 0.50);
grade 3/4 infection (two studies, N = 1663; main analysis: RR 1.04, 95% CI 0.91 to 1.19, P = 0.58; sensitivity analysis by random‐effects model: RR 1.16, 95% CI 0.78 to 1.72, P = 0.46).
We did not perform sensitivity analysis based on methodological quality of the studies for the above grade 3/4 AEs because all included studies were judged as high risk of bias for blinding regarding all outcomes except OS.
Subtype analysis
The subgroup analyses of grade 3/4 nausea/vomiting, diarrhoea, renal toxicity, and mucositis by dose of IDA showed no interaction between dose of IDA and effect size across all the subgroups (all P for subgroup differences > 0.05).
We did not perform subgroup analyses of any grade 3/4 AEs by age, cytogenetic risk stratification or AML subtype because no subgroup data were available.
Quality of life (QoL)
Data for QoL were not available.
Comparison 3: IDA versus DOX
Two studies enrolling 211 newly diagnosed AML patients compared IDA with DOX in induction therapy.
Primary outcome measures
Overall survival (OS)
Neither study reported OS data.
Disease‐free survival (DFS)
Bezwoda 1990 (N = 63) showed no statistically significant difference in DFS between patients with IDA and those with DOX in induction therapy (HR 0.62, 95% CI 0.34 to 1.14, P = 0.12).
Intragumtornchai 1999 did not report data on DFS.
Secondary outcome measures
Complete remission (CR)
Both studies reported on CR (N = 187).
The main analysis showed a statistically significantly improved CR for patients with IDA compared with those with DOX in induction therapy (RR 1.28, 95% CI 1.03 to 1.59, P = 0.02; I² for heterogeneity = 0%, P = 0.32).
Sensitivity analysis
A similar result was obtained by sensitivity analysis using random‐effects model (RR 1.29, 95% CI 1.04 to 1.60, P = 0.02; I² for heterogeneity = 0%, P = 0.32).
We did not perform sensitivity analysis based on methodological quality of the studies for CR rate because all included studies were judged as high risk of bias for blinding regarding all outcomes except OS.
Subgroup analysis
The subgroup analysis by dose of IDA (12 mg/m²/d or 20 mg/m²/d) showed no interaction between dose of IDA and effect size across all the subgroups. When the dose of IDA was 12 mg/m²/d, the RR was 1.43 (95% CI 1.06 to 1.95, P = 0.02; one study, 87 patients) (Intragumtornchai 1999). When the dose of IDA was 20 mg/m²/d, the RR was 1.15 (95% CI 0.85 to 1.57, P = 0.36; one study, 100 patients) (Bezwoda 1990) (P for subgroup differences = 0.32).
The subgroup analysis of CR by AML subtype (APL or non‐APL AML) showed no interaction between AML subtype and effect size across all the subgroups. In patients with APL, the RR was 1.00 (95% CI 0.50 to 2.00, P = 1.00; one study, 12 patients) (Bezwoda 1990). In patients with non‐APL AML, the RR was 1.16 (95% CI 0.83 to 1.62, P = 0.38; one study, 88 patients) (Bezwoda 1990) (P for subgroup differences = 0.71).
No data were available for analysing CR according to cytogenetic risk stratification and age.
Death on induction therapy
There were no data for death on induction therapy.
Relapse
Neither study reported relapse data.
Adverse events (AEs)
Bezwoda 1990 (N = 100) showed no statistically significant difference in grade 3/4 cardiac toxicity (RR 0.31, 95% CI 0.01 to 7.39, P = 0.47).
Intragumtornchai 1999 did not report any grade 3/4 AEs.
Quality of life (QoL)
Neither study reported data regarding QoL.
Comparison 4: IDA versus ZRB
Two studies with 1037 newly diagnosed AML patients assessed the effects of IDA versus ZRB in induction therapy.
Primary outcome measures
Overall survival (OS)
Neither study reported OS data.
Disease‐free survival (DFS)
Pignon 1996 (N =155) indicated no statistically significant difference in DFS between IDA and ZRB in induction therapy of patients with newly diagnosed AML (HR 1.25, 95% CI 0.83 to 1.88, P = 0.29).
Harousseau 1996 did not provide data for DFS.
Secondary outcome measures
Complete remission (CR)
Both studies reported CR data (N = 964). The main analysis showed no statistically significant difference in CR between arms (RR 1.04, 95% CI 0.96 to 1.13, P = 0.31; I² for heterogeneity = 74%, P = 0.05).
Sensitivity analysis
A similar result was obtained from the sensitivity analysis using random‐effects model (RR 1.09, 95% CI 0.89 to 1.33, P = 0.42; I² for heterogeneity = 74%, P = 0.05).
We did not perform sensitivity analysis based on methodological quality of the studies for CR because all included studies were judged as high risk of bias for blinding regarding all outcomes except OS.
Subgroup analysis
We did not conduct subgroup analysis of CR by dose of IDA because in both studies IDA was at 8 mg/m²/d.
We also did not conduct subgroup analyses of CR by cytogenetic risk stratification, age and AML subtype, because neither study provided data to analyse CR according to these characteristics.
Death on induction therapy
Both studies assessed death on induction therapy (N = 964). No statistically significant difference in the risk of death on induction therapy was found between arms in the main analysis (RR 0.75, 95% CI 0.50 to 1.13, P = 0.17; I² for heterogeneity = 24%, P = 0.25).
Sensitivity analysis
The same result was obtained in the sensitivity analysis by random‐effects model (RR 0.73, 95% CI 0.45 to 1.20, P = 0.21; I² for heterogeneity = 24%, P = 0.25).
We did not perform sensitivity analysis based on methodological quality of the studies for risk of death on induction therapy because all included studies were judged as high risk of bias for blinding regarding all outcomes except OS.
Subgroup analysis
We did not conduct subgroup analysis of death on induction therapy by dose of IDA because in both studies IDA was at 8 mg/m²/d.
We also did not perform subgroup analyses of death on induction therapy by cytogenetic risk stratification, age and AML subtype, because no data were available for these subgroup analyses.
Relapse
Neither study reported data regarding relapse.
Adverse events (AEs)
Pignon 1996 (N = 155) showed a statistically significantly lower risk of grade 3/4 mucositis with IDA versus ZRB (19 of 116 patients in the IDA arm versus 36 of 117 patients in the ZRB arm, P = 0.01). There were no statistically significant differences regarding grade 3/4 nausea/vomiting (26 of 116 patients in the IDA arm versus 40 of 117 patients in the ZRB arm, P = 0.05), diarrhoea (13 of 116 patients in the IDA arm versus 14 of 117 patients in the ZRB arm, P = 0.86), and hepatic toxicity (14 of 116 patients in the IDA arm versus 10 of 117 patients in the ZRB arm, P = 0.38).
Harousseau 1996 did not assess any grade 3/4 AEs.
Quality of life (QoL)
Neither study reported data regarding QoL.
Discussion
Summary of main results
In this systematic review and meta‐analysis, we assessed the efficacy and safety of idarubicin (IDA) versus other anthracyclines in induction therapy of patients with newly diagnosed acute myeloid leukaemia (AML). The consolidation treatments adopted in the included studies were comparable and had no impact on the results. We obtained the following results from the meta‐analyses.
Compared with daunorubicin (DNR) in induction therapy of newly diagnosed AML, IDA can prolong overall survival (OS) and disease‐free survival (DFS), increase complete remission (CR) rate and reduce relapse rate, although it may increase the risks of death on induction therapy and grade 3/4 mucositis. Subgroup analyses identified that cytogenetic risk stratification might be an influencing factor for the CR rate effect, and dose of IDA, total dose of IDA and dose ratio might be influencing factors for the relapse rate effect of IDA versus DNR. Additionally, IDA was associated with a statistically significantly reduced risk of grade 3/4 hepatic toxicity when the total dose of DNR was more than 180 mg/m². There were no statistically significant differences between arms regarding other grade 3/4 adverse events, including nausea/vomiting, alopecia, diarrhoea, renal toxicity, cardiac toxicity, skin toxicity, cental neurotoxicity, bleeding, stomatitis, infection and sepsis. The effect of IDA versus DNR on quality of life (QoL) was not reported.
Compared with mitoxantrone (MIT) in induction therapy of newly diagnosed AML, there were no statistically significant differences in OS, DFS, CR rate, the risks of death on induction therapy, relapse and grade 3/4 adverse events (including nausea/vomiting, diarrhoea, mucositis, hepatic toxicity, renal toxicity and infection) between patients with IDA and those with MIT. The effect of IDA versus MIT on QoL was not reported.
Compared with doxorubicin (DOX) in induction therapy of newly diagnosed AML, IDA can increase the CR rate. There were no statistical differences between arms in DFS and the risk of grade 3/4 cardiac toxicity. The following outcomes were not reported for IDA versus DOX: OS, the risk of death on induction therapy, relapse rate and QoL.
Compared with zorubicin (ZRB) in induction therapy of newly diagnosed AML, IDA can reduce the risk of grade 3/4 mucositis. There were no statistical differences between arms in DFS, CR rate and the risk of death on induction therapy. The following outcomes were not reported for IDA versus ZRB: OS, relapse rate, the risk of grade 3/4 cardiotoxicity and QoL.
Overall completeness and applicability of evidence
In this systematic review and meta‐analysis, IDA was compared to DNR, MIT, DOX and ZRB in induction therapy of newly diagnosed AML patients, respectively. These comparisons included all mainstream anthracyclines that can be used in induction therapy for newly diagnosed AML patients. The inclusion criteria of participants for all included randomised controlled trials (RCTs) are consistent with clinical practice in "real‐world" conditions. All these aspects increase the applicability of this systematic review and meta‐analysis.
However, OS data were not provided in, or not able to be extracted from, six out of 18 studies in the meta‐analysis for IDA versus DNR, two out of eight studies in the meta‐analysis for IDA versus MIT, all studies in the meta‐analysis for IDA versus DOX and for IDA versus ZRB. DFS data were not provided in, or not able to be extracted from, 10 out of 18 studies in the meta‐analysis for IDA versus DNR, four out of eight studies in the meta‐analysis for IDA versus MIT, one out of two studies in the meta‐analysis for IDA versus DNR, one out of two studies in the meta‐analysis for IDA versus MIT. None of the included studies reported on QoL. Although the overall completeness of evidence was lower by these factors, this systematic review and meta‐analysis can also provide valuable suggestions in choosing an appropriate anthracycline for induction therapy for newly diagnosed AML patients.
The current National Comprehensive Cancer Network (NCCN) guideline suggests that either DNR or IDA is recommended in induction therapy of newly diagnosed AML patients. Our systematic review and meta‐analysis not only compared IDA versus DNR, but also compared IDA with other anthracyclines. This comprehensive comparison might be more useful in helping clinicians when making choices of treatment for induction therapy.
Quality of the evidence
All included studies were reported as RCTs. Although only one (Jia 2011) of the included studies reported the method of random sequence generation, other studies probably had an adequate sequence generation because they did not explicitly state they were quasi‐randomised, or were very old. Three (Mandelli 1991; Mandelli 2009; Ohtake 2011) studies adequately concealed allocation. The unclear allocation concealment could introduce selection bias.
Five studies (Gardin 2007; Lee 2012; Ohtake 2011; Pautas 2010; Récher 2014) were reported as open‐label studies and none of the studies provided information for blinding of outcome assessors. This might lead to performance or detection bias for all outcomes except OS.
Fifteen studies included all randomised patients in the analysis (Archimbaud 1999; Creutzig 2001; Creutzig 2013; Eridani 1989; Feng 2010; Gardin 2007; Indrak 2001; Jia 2011; Lee 2012; Mandelli 1991; Mandelli 2009; Ohtake 2011; Reiffers 1996; Wang 2011; Wiernik 1992). Eight studies had missing data which were balanced in numbers and reasons between arms (Beksac 1998; Bezwoda 1990; Masaoka 1996; Pautas 2010; Pignon 1996; Récher 2014; Rowe 2004; Vogler 1992). In two studies (Berman 1991; Harousseau 1996), the percentage of missing data was less than 10% of randomised patients. We judged these 25 studies as low risk of attrition bias. In one other study (Intragumtornchai 1999), the proportion of missing outcomes reached 19% of randomised patients and we assessed this might induce attrition bias. In one study published only as abstracts (De Moerloose 2011), there was insufficient information about statistical methods and patient analyses, therefore, we judged the risk of attrition bias as unclear for this study.
Three studies (Gardin 2007; Ohtake 2011; Pautas 2010) reported all pre‐planned outcomes in the protocol and we judged them as low risk of reporting bias. The protocols of De Moerloose 2011, Mandelli 2009 and Récher 2014 did not provide information about outcomes that would be assessed and therefore we judged the risk of reporting bias as unclear in the three studies. In Lee 2012, only a few outcomes that were pre‐defined in the protocol were reported and we judged the study as high risk of reporting bias. Protocols for other studies were not available and we judged the risk of reporting bias as unclear for these studies.
Additionally, in three studies (Masaoka 1996; Pignon 1996; Vogler 1992), baseline characteristics of patients were not balanced between arms and we judged these studies as at high risk of other potential sources of bias.
Collectively, the comparison between IDA and DNR provides high quality of evidence for OS and moderate quality of evidence for other outcomes because lack of blinding will influence all outcomes except OS (Table 1). In the comparison between IDA and MIT, the quality of evidence for OS is high, the quality of evidence for DFS is low due to lack of blinding and the small number of patients; the quality of evidence for CR, death on induction therapy and relapse is moderate because of lack of blinding; and the quality of evidence for grade 3/4 cardiotoxicity is low because of lack of blinding and because only one trial was included (Table 2). In the comparison between IDA and DOX, the quality of evidence for DFS is low due to lack of binding and because only one trial was included; the quality of evidence for CR is low because of lack of binding and a large loss to follow‐up in one study; and the quality of evidence for grade 3/4 cardiotoxicity is very low because of lack of blinding, the inclusion of only one study and the relatively few events producing a wide confidence interval around the effect estimate (Table 3). In the comparison between IDA and ZRB, the quality of evidence for DFS is low because of lack of binding and because only one study was included; the quality of evidence for CR is low because of lack of binding and unexplained substantial heterogeneity between trials; and the quality of evidence for death on induction therapy is moderate because of lack of blinding (Table 4).
In addition, the main aim of this systematic review is to assess the efficacy of different anthracyclines in induction therapy of AML on long‐term survival, but an additional concern is whether consolidation treatments will impact on the results. Twelve of the included studies (Archimbaud 1999; Beksac 1998; Creutzig 2001; De Moerloose 2011; Eridani 1989; Indrak 2001; Lee 2012; Mandelli 1991; Mandelli 2009; Pignon 1996; Récher 2014; Rowe 2004) reported that patients received the same consolidation treatment after achieving CR; six studies (Berman 1991; Bezwoda 1990; Pautas 2010; Reiffers 1996; Vogler 1992; Wiernik 1992) adopted a consistent drug in consolidation therapy as in induction therapy; four studies (Creutzig 2013; Gardin 2007; Harousseau 1996; Ohtake 2011) re‐randomised patients after achieving CR. Five studies (Feng 2010; Intragumtornchai 1999; Jia 2011; Masaoka 1996; Wang 2011) that did not report the method of consolidation therapy only provided results on CR and AEs. Collectively, the consolidation treatments would not impact on the results in all these conditions.
Potential biases in the review process
To prevent potential bias, we only included RCTs in this review. In addition, we performed a comprehensive retrieval strategy to identify studies relevant to the review, including searching databases, conference proceedings, trial registries, and handsearching references of all identified trials and relevant review articles and current treatment guidelines, with no restriction on location or language. Furthermore, to avoid bias, all relevant processes (searching, study selection, data collection and analysis) were performed by two review authors. Therefore, we are not aware of any obvious defects in our review process. However, we cannot exclude the potential risk of publication bias. For example, although there were enough studies to perform a funnel plot for CR in the comparison of IDA versus DNR, the funnel plot was not symmetrical, indicating that there is potential risk of publication bias for the benefit of IDA. Additionally, for some outcomes there were not enough studies to draw a funnel plot, therefore, the potential risk of publication bias cannot be excluded as more studies may be needed for analysis.
Additionally, we did not perform all the subgroup analysis exactly as stated in the protocol (see 'Differences between protocol and review' section). Firstly, we had intended to perform subgroup analysis by anthracycline types of control intervention, but in order to avoid clinical heterogeneity, instead we undertook separate meta‐analyses for IDA versus different anthracyclines. Secondly, we added a post‐protocol subgroup analysis by total dose of DNR (< 180 mg/m² or ≥ 180 mg/m²). Thirdly, we planned to perform subgroup analysis by age (< 60 years or ≥60 years) in the protocol, but actually we undertook amore detailed subgroup analysis by age (< 15 years or ≥ 15 years to < 60 years or ≥60 years).
Agreements and disagreements with other studies or reviews
The role of IDA in induction therapy has been extensively investigated by RCTs and meta‐analyses since its approval for use. The earliest meta‐analysis using individual patient data was published by the AML Collaborative Group (AML Collaborative Group 1998). The authors compared IDA versus DNR or other anthracyclines when used with cytarabine (Ara‐C) as induction therapy for newly diagnosed AML. Based on five trials with 1052 patients, it showed that IDA was superior to DNR in prolonging OS and increasing CR rate, but had a non‐significant benefit in DFS. Based on one trial with 100 patients comparing IDA versus DOX, it showed that there were no significant differences in OS, DFS and CR between IDA with DOX. Based on another trial with 745 patients comparing IDA versus ZRB, it showed that there were also no significant differences in OS, DFS and CR between IDA with ZRB. Our systematic review summarises all the evidence currently available, including 18 RCTs assessing IDA versus DNR, two assessing IDA versus DOX and two assessing IDA versus ZRB, as well as eight RCTs comparing IDA versus MIT. The number of participants is larger and the outcomes are more comprehensive compared with previous reviews.
Another systematic review and meta‐analysis recently published by Teuffel et al. aimed to compare the efficacy of different types of anthracyclines and different dosing of anthracycline schedules for induction therapy in children and young adult AML patients (Teuffel 2013). The main objectives of this meta‐analysis were remission failure rate, death on induction therapy and overall mortality, but did not report any data about survival. For the comparison of IDA versus DNR, it concluded that IDA reduced remission failure rate, but did not alter the rate of death during induction, or overall mortality. The conclusion regarding remission failure rate was based on eight RCTs involving 3382 patients, death during induction therapy based on six RCTs with 3205 patients, and overall mortality only based on four trials with 2973 patients. In our systematic‐review, we assessed CR instead of remission failure rate and found a statistically significantly improved CR based on 19 RCTs with 6767 patients. Regarding the outcome death during induction, our conclusion was based on 14 RCTs with 6349 patients. Overall mortality was also not assessed in our review, but we analysed OS and DFS based on more RCTs. For the comparison of IDA versus MIT, the meta‐analysis by Teuffel et al identified six RCTs and showed no differences for any outcome measures, which was similar to our results. Collectively, compared with the meta‐analysis published by Teuffel et al. our meta‐analysis is based on more participants and the outcomes of our meta‐analysis are more comprehensive.
Another meta‐analysis published by Wang et al. compared IDA + Ara‐C regimen with DNA + Ara‐C regimen as induction therapy for patients with newly diagnosed AML (Wang 2013). It included 10 RCTs with 4060 patients and found that IDA + Ara‐C regimen was associated with a significant advantage in CR, event‐free survival and OS, but not in DFS. The results were similar to what we found in this meta‐analysis. However, the authors did not compare IDA with other anthracyclines (Wang 2013).
Authors' conclusions
Implications for practice.
Compared with daunorubicin (DNR) in induction therapy of patients with newly diagnosed acute myeloid leukaemia (AML), idarubicin (IDA) can prolong overall survival (OS) and disease‐free survival (DFS), increase the complete remission (CR) rate, and reduce relapse rate, although it may increase the risks of death on induction therapy and grade 3/4 mucositis. The currently available evidence suggests that there is no evidence for a difference between IDA and mitoxantrone (MIT) used in induction therapy. There is insufficient evidence regarding IDA versus doxorubicin (DOX) and IDA versus zorubicin (ZRB) to make final conclusions. Additionally, there is no evidence for the difference on the effect of IDA compared with DNR, MIT, DOX or ZRB on quality of life (QoL).The consolidation treatments adopted in the included studies were comparable and had no impact on the results.
Implications for research.
More randomised controlled trials (RCTs) are needed to evaluate the role of IDA versus DNR for induction therapy in specific AML patient subgroups, such as children or elderly patients, those at different cytogenetic risk stratifications and those with different AML subtypes. In addition, the optimal dose and schedule of IDA as well as DNR should be evaluated in RCTs and more RCTs are needed to assess the efficacy of IDA versus other anthracyclines, such as MIT, DOX and ZRB. Finally, future RCTs should evaluate QoL since QoL is becoming an increasingly important consideration for clinicians when selecting therapy strategies for patients with cancers.
Acknowledgements
We are grateful to Ina Monsef, Nicole Skoetz, Andrea Will, Oliver Blank and Michaela Rancea from the Cochrane Haematological Malignancies Group for their helpful comments in improving the review.
Appendices
Appendix 1. CENTRAL search strategy
| ID | Search |
| #1 | MeSH descriptor Leukemia, Myeloid, Acute explode all trees |
| #2 | MeSH descriptor Leukemia, Myeloid explode all trees |
| #3 | MeSH descriptor Acute Disease explode all trees |
| #4 | (#2 AND #3) |
| #5 | (acut* or akut* or agud* or aigu*) |
| #6 | ((myelo* or mielo* or nonlympho* or granulocytic*) and (leuk*em* or leuc*)) |
| #7 | (#5 AND #6) |
| #8 | aml* |
| #9 | anll |
| #10 | (#1 OR #4 OR #7 OR #8 OR #9) |
| #11 | MeSH descriptor Idarubicin explode all trees |
| #12 | Idarubicin* |
| #13 | idamycin* |
| #14 | idaralem* |
| #15 | zavedos* |
| #16 | (desmethoxydaunorubicin* or demethoxydaunorubicin*) |
| #17 | (imi‐30* or imi30*) |
| #18 | (nsc‐256439* or nsc256439*) |
| #19 | dmdr* |
| #20 | idr* |
| #21 | ida* |
| #22 | (#11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21) |
| #23 | (#10 AND #22) |
Appendix 2. MEDLINE search strategy
OVID MEDLINE search strategy
| # | Searches |
| 1 | exp Leukemia, Myeloid, Acute/ |
| 2 | leukemia, myeloid/ |
| 3 | acute disease/ |
| 4 | 2 and 3 |
| 5 | (acut$ or akut$ or agud$ or aigu$).tw,kf,ot. |
| 6 | ((myelo$ or mielo$ or nonlympho$ or granulocytic$) and (leuk?em$ or leuc$)).tw,kf,ot. |
| 7 | 5 and 6 |
| 8 | aml.tw,kf,ot. |
| 9 | anll.tw,kf,ot. |
| 10 | 1 or 4 or 7 or 8 or 9 |
| 11 | Idarubicin/ |
| 12 | idarubicin$.tw,kf,ot. |
| 13 | idamycin$.tw,kf,ot. |
| 14 | idaralem$.tw,kf,ot. |
| 15 | zavedos$.tw,kf,ot. |
| 16 | (desmethoxydaunorubicin$ or demethoxydaunorubicin$).tw,kf,nm,ot. |
| 17 | (imi‐30$ or imi30$).tw,kf,nm,ot. |
| 18 | (nsc‐256439$ or nsc256439$).tw,kf,nm,ot. |
| 19 | dmdr$.tw,kf,nm,ot. |
| 20 | idr$.tw,kf,nm,ot. |
| 21 | ida$.tw,kf,nm,ot. |
| 22 | or/11‐21 |
| 23 | 10 and 22 |
| 24 | randomised controlled trial.pt. |
| 25 | controlled clinical trial.pt. |
| 26 | randomised.ab. |
| 27 | placebo.ab. |
| 28 | drug therapy.fs. |
| 29 | randomly.ab. |
| 30 | trial.ab. |
| 31 | groups.ab. |
| 32 | or/24‐31 |
| 33 | humans.sh. |
| 34 | 32 and 33 |
| 35 | 10 and 22 and 34 |
Appendix 3. EMBASE search strategy
| # | Searches |
| 1 | 'ACUTE GRANULOCYTIC LEUKEMIA':exp |
| 2 | 'MYELOID LEUKEMIA':de |
| 3 | 'ACUTE DISEASE':de |
| 4 | #2 AND #3 |
| 5 | acut*:ab,ti,tt OR akut*:ab,ti,tt OR agud*:ab,ti,tt OR aigu*:ab,ti,tt |
| 6 | ((myelo*:ab,ti,tt OR mielo*:ab,ti,tt OR nonlympho*:ab,ti,tt OR granulocytic*:ab,ti,tt) AND (leuk*em*:ab,ti,tt OR leuc*:ab,ti,tt)) |
| 7 | #5 AND #6 |
| 8 | 'aml':ab,ti,tt |
| 9 | 'anll':ab,ti,tt |
| 10 | #1 or #4 or #7 or #8 or #9 |
| 11 | 'IDARUBICIN':de |
| 12 | idarubicin*:ab,ti,tt |
| 13 | idamycin*:ab,ti,tt |
| 14 | idaralem*:ab,ti,tt |
| 15 | zavedos*:ab,ti,tt |
| 16 | desmethoxydaunorubicin*:ab,ti,tt OR demethoxydaunorubicin*:ab,ti,tt |
| 17 | imi‐30*:ab,ti,tt OR imi30*:ab,ti,tt |
| 18 | nsc‐256439*:ab,ti,tt OR nsc256439*:ab,ti,tt |
| 19 | dmdr*:ab,ti,tt |
| 20 | idr*:ab,ti,tt |
| 21 | ida*:ab,ti,tt |
| 22 | #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 |
| 23 | #10 AND #22 |
| 24 | 'randomised controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp |
| 25 | random*:ab,ti OR placebo*:ab,ti OR allocat*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR trial:ti OR (doubl* NEXT/1 blind*):ab,ti |
| 26 | #24 OR #25 |
| 27 | 'animal'/de OR 'animal experiment'/de OR 'nonhuman'/de |
| 28 | 'human'/de |
| 29 | #27 AND #28 |
| 30 | #27 NOT #29 |
| 31 | #26 NOT #30 |
| 32 | #10 AND #22 AND #31 |
Appendix 4. CBM search strategy
| # | Searches |
| 1 | myeloid leukemia AND idarubicin |
| 2 | myeloid leukemia AND demethoxydaunorubicin |
Appendix 5. Other search strategy
| # | Searches |
| 1 | myeloid leukemia AND idarubicin |
| 2 | myeloid leukemia AND idamycin |
| 3 | myeloid leukemia AND idaralem |
| 4 | myeloid leukemia AND zavedos |
| 5 | myeloid leukemia AND desmethoxydaunorubicin |
| 6 | myeloid leukemia AND demethoxydaunorubicin |
| 7 | myeloid leukaemia AND idarubicin |
| 8 | myeloid leukaemia AND idamycin |
| 9 | myeloid leukaemia AND idaralem |
| 10 | myeloid leukaemia AND zavedos |
| 11 | myeloid leukaemia AND desmethoxydaunorubicin |
| 12 | myeloid leukaemia AND demethoxydaunorubicin |
Data and analyses
Comparison 1. IDA versus DNR.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 OS‐overall analysis | 12 | 5976 | Hazard Ratio (Fixed, 95% CI) | 0.90 [0.84, 0.96] |
| 2 OS‐sensitivity analysis by random‐effects model | 12 | 5976 | Hazard Ratio (Random, 95% CI) | 0.89 [0.81, 0.97] |
| 3 OS‐sensitivity analysis by excluding studies with high risk of bias | 11 | 5758 | Hazard Ratio (Fixed, 95% CI) | 0.90 [0.85, 0.96] |
| 4 OS‐subgroup analysis by dose of IDA | 12 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 4.1 8 mg/m²/d | 2 | 1038 | Hazard Ratio (Fixed, 95% CI) | 0.85 [0.74, 0.99] |
| 4.2 9 mg/m²/d | 1 | 416 | Hazard Ratio (Fixed, 95% CI) | 0.90 [0.73, 1.12] |
| 4.3 10 mg/m²/d | 1 | 1438 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.82, 1.08] |
| 4.4 12 mg/m²/d | 7 | 2870 | Hazard Ratio (Fixed, 95% CI) | 0.92 [0.84, 1.00] |
| 4.5 13 mg/m²/d | 1 | 214 | Hazard Ratio (Fixed, 95% CI) | 0.70 [0.52, 0.94] |
| 5 OS‐subgroup analysis by total dose of DNR | 12 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 5.1 < 180 mg/m2 | 8 | 3109 | Hazard Ratio (Fixed, 95% CI) | 0.89 [0.82, 0.97] |
| 5.2 ≧ 180 mg/m2 | 4 | 2867 | Hazard Ratio (Fixed, 95% CI) | 0.91 [0.82, 1.00] |
| 6 OS‐subgroup analysis by dose of IDA versus dose of DNR | 12 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 6.1 9 mg/m²/d IDA versus 45 mg/m²/d DNR | 1 | 416 | Hazard Ratio (Fixed, 95% CI) | 0.90 [0.73, 1.12] |
| 6.2 12 mg/m²/d IDA versus 45 mg/m²/d DNR | 3 | 701 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.81, 1.09] |
| 6.3 13 mg/m²/d IDA versus 45 mg/m²/d DNR | 1 | 214 | Hazard Ratio (Fixed, 95% CI) | 0.70 [0.52, 0.94] |
| 6.4 8 mg/m²/d IDA versus 50 mg/m²/d DNR | 1 | 220 | Hazard Ratio (Fixed, 95% CI) | 0.86 [0.65, 1.13] |
| 6.5 10 mg/m²/d IDA versus 50 mg/m²/d DNR | 1 | 1438 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.82, 1.08] |
| 6.6 12 mg/m²/d IDA versus 50 mg/m²/d DNR | 2 | 1180 | Hazard Ratio (Fixed, 95% CI) | 0.97 [0.83, 1.14] |
| 6.7 8 mg/m²/d IDA versus 60 mg/m²/d DNR | 1 | 818 | Hazard Ratio (Fixed, 95% CI) | 0.85 [0.71, 1.02] |
| 6.8 12 mg/m²/d IDA versus 80 mg/m²/d DNR | 2 | 989 | Hazard Ratio (Fixed, 95% CI) | 0.83 [0.71, 0.98] |
| 7 OS‐subgroup analysis by age | 6 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 7.1 < 15 years | 1 | 521 | Hazard Ratio (Fixed, 95% CI) | 1.07 [0.78, 1.47] |
| 7.2 ≥ 15 years to < 60 years | 3 | 2376 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.80, 0.98] |
| 7.3 ≥ 60 years | 2 | 527 | Hazard Ratio (Fixed, 95% CI) | 0.95 [0.79, 1.15] |
| 8 OS‐subgroup analysis by cytogenetic risk stratification | 1 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 8.1 Favourable | 1 | 123 | Hazard Ratio (Fixed, 95% CI) | 0.51 [0.23, 1.12] |
| 8.2 Intermediate | 1 | 468 | Hazard Ratio (Fixed, 95% CI) | 0.70 [0.54, 0.90] |
| 8.3 Adverse | 1 | 174 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.67, 1.31] |
| 9 DFS‐overall analysis | 8 | 3070 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.81, 0.96] |
| 10 DFS‐sensitivity analysis by random‐effects model | 8 | 3070 | Hazard Ratio (Random, 95% CI) | 0.88 [0.80, 0.96] |
| 11 DFS‐subgroup analysis by dose of IDA | 8 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 11.1 8 mg/m²/d | 2 | 816 | Hazard Ratio (Fixed, 95% CI) | 0.82 [0.69, 0.98] |
| 11.2 10 mg/m²/d | 1 | 975 | Hazard Ratio (Fixed, 95% CI) | 0.85 [0.71, 1.02] |
| 11.3 12 mg/m²/d | 4 | 1145 | Hazard Ratio (Fixed, 95% CI) | 0.96 [0.85, 1.08] |
| 11.4 13 mg/m²/d | 1 | 134 | Hazard Ratio (Fixed, 95% CI) | 0.64 [0.43, 0.94] |
| 12 DFS‐subgroup analysis by total dose of DNR | 8 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 12.1 < 180 mg/m2 | 6 | 1573 | Hazard Ratio (Fixed, 95% CI) | 0.84 [0.75, 0.94] |
| 12.2 ≧ 180 mg/m2 | 2 | 1497 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.83, 1.07] |
| 13 DFS‐subgroup analysis by dose of IDA versus dose of DNR | 8 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 13.1 12 mg/m²/d IDA versus 45 mg/m²/d DNR | 3 | 322 | Hazard Ratio (Fixed, 95% CI) | 0.90 [0.74, 1.09] |
| 13.2 13 mg/m²/d IDA versus 45 mg/m²/d DNR | 1 | 134 | Hazard Ratio (Fixed, 95% CI) | 0.64 [0.43, 0.94] |
| 13.3 8 mg/m²/d IDA versus 50 mg/m²/d DNR | 1 | 142 | Hazard Ratio (Fixed, 95% CI) | 0.78 [0.58, 1.04] |
| 13.4 10 mg/m²/d IDA versus 50 mg/m²/d DNR | 1 | 975 | Hazard Ratio (Fixed, 95% CI) | 0.85 [0.71, 1.02] |
| 13.5 12 mg/m²/d IDA versus 50 mg/m²/d DNR | 1 | 823 | Hazard Ratio (Fixed, 95% CI) | 1.0 [0.85, 1.17] |
| 13.6 8 mg/m²/d IDA versus 60 mg/m²/d DNR | 1 | 674 | Hazard Ratio (Fixed, 95% CI) | 0.84 [0.68, 1.05] |
| 14 DFS‐subgroup analysis by cytogenetic risk stratification | 1 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 14.1 Favourable | 1 | 118 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.47, 1.64] |
| 14.2 Intermediate | 1 | 393 | Hazard Ratio (Fixed, 95% CI) | 0.73 [0.56, 0.97] |
| 14.3 Adverse | 1 | 113 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.62, 1.41] |
| 15 CR‐overall analysis | 18 | 6692 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [1.01, 1.07] |
| 16 CR‐sensitivity analysis by random‐effects model | 18 | 6692 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [1.01, 1.10] |
| 17 CR‐subgroup analysis by dose of IDA | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 8 mg/m²/d | 3 | 1083 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.97, 1.09] |
| 17.2 9 mg/m²/d | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.92, 1.29] |
| 17.3 10 mg/m²/d | 2 | 1462 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.92, 1.06] |
| 17.4 12 mg/m²/d | 10 | 3446 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [1.01, 1.09] |
| 17.5 13 mg/m²/d | 1 | 214 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.94, 1.42] |
| 18 CR‐subgroup analysis by total dose of DNR | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 < 180 mg/m2 | 13 | 3671 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [1.01, 1.11] |
| 18.2 ≥ 180 mg/m2 | 5 | 3021 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.98, 1.06] |
| 19 CR‐subgroup analysis by dose of IDA versus dose of DNR | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 8 mg/m²/d IDA versus 25 mg/m²/d DNR | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.64, 2.03] |
| 19.2 12 mg/m²/d IDA versus 40 mg/m²/d DNR | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.88, 2.43] |
| 19.3 9 mg/m²/d IDA versus 45 mg/m²/d DNR | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.92, 1.29] |
| 19.4 10 mg/m²/d IDA versus 45 mg/m²/d DNR | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.38 [0.90, 12.74] |
| 19.5 12 mg/m²/d IDA versus 45 mg/m²/d DNR | 3 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.97, 1.31] |
| 19.6 13 mg/m²/d IDA versus 45 mg/m²/d DNR | 1 | 214 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.94, 1.42] |
| 19.7 8 mg/m²/d IDA versus 50 mg/m²/d DNR | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.91, 1.35] |
| 19.8 10 mg/m²/d IDA versus 50 mg/m²/d DNR | 1 | 1438 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.05] |
| 19.9 12 mg/m²/d IDA versus 50 mg/m²/d DNR | 2 | 1177 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.97, 1.10] |
| 19.10 8 mg/m²/d IDA versus 60 mg/m²/d DNR | 1 | 818 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| 19.11 12 mg/m²/d IDA versus 60 mg/m²/d DNR | 1 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.91, 1.07] |
| 19.12 12 mg/m²/d IDA versus 80 mg/m²/d DNR | 2 | 989 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.99, 1.11] |
| 19.13 12 mg/m²/d IDA versus 90 mg/m²/d DNR | 1 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.84, 1.16] |
| 20 CR‐subgroup analysis by age | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 < 15 years | 1 | 521 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.93, 1.05] |
| 20.2 ≥ 15 years to < 60 years | 8 | 3294 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [1.00, 1.08] |
| 20.3 ≥ 60 years | 6 | 751 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.95, 1.22] |
| 21 CR‐subgroup analysis by cytogenetic risk stratification | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.1 Favourable | 2 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.88, 1.01] |
| 21.2 Intermediate | 3 | 1080 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.98, 1.11] |
| 21.3 Adverse | 3 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.97, 1.28] |
| 22 Death on induction therapy‐overall analysis | 14 | 6349 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.01, 1.36] |
| 23 Death on induction therapy‐sensitivity analysis by random‐effects model | 14 | 6349 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.97, 1.41] |
| 24 Death on induction therapy‐subgroup analysis by dose of IDA | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 24.1 8 mg/m²/d | 2 | 1038 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.79, 1.83] |
| 24.2 9 mg/m²/d | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.51, 1.65] |
| 24.3 10 mg/m²/d | 2 | 1462 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.83, 1.41] |
| 24.4 12 mg/m²/d | 8 | 3225 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.04, 1.63] |
| 24.5 13 mg/m²/d | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.67, 1.96] |
| 25 Death on induction therapy‐subgroup analysis by total dose of DNR | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 25.1 < 180 mg/m2 | 10 | 3485 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.00, 1.38] |
| 25.2 ≧ 180 mg/m2 | 4 | 2864 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.83, 1.70] |
| 26 Death on induction therapy‐subgroup analysis by dose of IDA versus dose of DNR | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 26.1 9 mg/m²/d IDA versus 45 mg/m²/d DNR | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.51, 1.65] |
| 26.2 10 mg/m²/d IDA versus 45 mg/m²/d DNR | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.08, 1.41] |
| 26.3 12 mg/m²/d IDA versus 45 mg/m²/d DNR | 3 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.98, 1.70] |
| 26.4 13 mg/m²/d IDA versus 45 mg/m²/d DNR | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.67, 1.96] |
| 26.5 8 mg/m²/d IDA versus 50 mg/m²/d DNR | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.78, 2.48] |
| 26.6 10 mg/m²/d IDA versus 50 mg/m²/d DNR | 1 | 1438 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.87, 1.48] |
| 26.7 12 mg/m²/d IDA versus 50 mg/m²/d DNR | 2 | 1177 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [1.04, 3.53] |
| 26.8 8 mg/m²/d IDA versus 60 mg/m²/d DNR | 1 | 818 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.56, 1.91] |
| 26.9 12 mg/m²/d IDA versus 60 mg/m²/d DNR | 1 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.59, 5.04] |
| 26.10 12 mg/m²/d IDA versus 80 mg/m²/d DNR | 2 | 989 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.42, 1.42] |
| 27 Death on induction therapy‐subgroup analysis by age | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 27.1 < 15 years | 1 | 521 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.44, 4.24] |
| 27.2 ≥ 15 years to < 60 years | 4 | 2390 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.85, 1.37] |
| 27.3 ≥ 60 years | 2 | 426 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.53, 1.60] |
| 28 Relapse‐overall analysis | 4 | 1091 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.80, 0.98] |
| 29 Relapse‐sensitivity analysis by random‐effects model | 4 | 1091 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.71, 1.01] |
| 30 Relapse‐subgroup analysis by dose of IDA | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 30.1 8 mg/m²/d | 1 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.66, 0.86] |
| 30.2 12 mg/m²/d | 3 | 960 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.81, 1.04] |
| 31 Relapse‐subgroup analysis by total dose of DNR | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 31.1 < 180 mg/m2 | 2 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.65, 0.85] |
| 31.2 ≧ 180 mg/m2 | 2 | 820 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.84, 1.13] |
| 32 Relapse‐subgroup analysis by dose of IDA versus dose of DNR | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 32.1 12 mg/m²/d IDA versus 45 mg/m²/d DNR | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.56, 0.93] |
| 32.2 8 mg/m²/d IDA versus 50 mg/m²/d DNR | 1 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.66, 0.86] |
| 32.3 12 mg/m²/d IDA versus 80 mg/m²/d DNR | 2 | 820 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.84, 1.13] |
| 33 Nausea/vomiting grade 3/4‐overall analysis | 4 | 622 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.73, 1.73] |
| 34 Nausea/vomiting grade 3/4‐sensitivity analysis by random‐effects model | 4 | 622 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.69, 1.65] |
| 35 Nausea/vomiting grade 3/4‐subgroup analysis by dose of IDA | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 35.1 8 mg/m²/d | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.51, 2.73] |
| 35.2 12 mg/m²/d | 3 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.67, 1.82] |
| 36 Nausea/vomiting grade 3/4‐subgroup analysis by dose of IDA versus dose of DNR | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 36.1 12 mg/m²/d IDA versus 40 mg/m²/d DNR | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.35, 1.39] |
| 36.2 12 mg/m²/d IDA versus 45 mg/m²/d DNR | 1 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.15 [0.55, 8.39] |
| 36.3 8 mg/m²/d IDA versus 50 mg/m²/d DNR | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.51, 2.73] |
| 36.4 12 mg/m²/d IDA versus 50 mg/m²/d DNR | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.58, 3.50] |
| 37 Alopecia grade 3/4‐overall analysis | 4 | 715 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.90, 1.31] |
| 38 Alopecia grade 3/4‐sensitivity analysis by random‐effects model | 4 | 715 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.86, 1.35] |
| 39 Alopecia grade 3/4‐subgroup analysis by dose of IDA | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 39.1 8 mg/m²/d | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.77, 1.30] |
| 39.2 12 mg/m²/d | 2 | 281 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.92, 2.04] |
| 39.3 13 mg/m²/d | 1 | 214 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.69, 1.41] |
| 40 Alopecia grade 3/4‐subgroup analysis by dose of IDA versus dose of DNR | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 40.1 12 mg/m²/d IDA versus 40 mg/m²/d DNR | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.34, 1.89] |
| 40.2 12 mg/m²/d IDA versus 45 mg/m²/d DNR | 1 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.01, 2.51] |
| 40.3 13 mg/m²/d IDA versus 45 mg/m²/d DNR | 1 | 214 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.69, 1.41] |
| 40.4 8 mg/m²/d IDA versus 50 mg/m²/d DNR | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.77, 1.30] |
| 41 Diarrhoea grade 3/4‐overall analysis | 3 | 502 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.68, 2.28] |
| 42 Diarrhoea grade 3/4‐sensitivity analysis by random‐effects model | 3 | 502 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.68, 2.29] |
| 43 Diarrhoea grade 3/4‐subgroup analysis by dose of IDA | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 43.1 8 mg/m²/d | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.14, 6.72] |
| 43.2 12 mg/m²/d | 2 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.68, 2.43] |
| 44 Diarrhoea grade 3/4‐subgroup analysis by dose of IDA versus dose of DNR | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 44.1 12 mg/m²/d IDA versus 40 mg/m²/d DNR | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.30] |
| 44.2 12 mg/m²/d IDA versus 45 mg/m²/d DNR | 1 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.68, 2.52] |
| 44.3 8 mg/m²/d IDA versus 50 mg/m²/d DNR | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.14, 6.72] |
| 45 Hepatic toxicity grade 3/4‐overall analysis | 5 | 1226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.59, 1.20] |
| 46 Hepatic toxicity grade 3/4‐sensitivity analysis by random‐effects model | 5 | 1226 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.57, 1.52] |
| 47 Hepatic toxicity grade 3/4‐subgroup analysis by dose of IDA | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 47.1 8 mg/m²/d | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.44, 2.13] |
| 47.2 12 mg/m²/d | 4 | 1006 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.54, 1.21] |
| 48 Hepatic toxicity grade 3/4‐subgroup analysis by total dose of DNR | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 48.1 < 180 mg/m2 | 4 | 743 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.75, 2.07] |
| 48.2 ≧ 180 mg/m2 | 1 | 483 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.32, 0.93] |
| 49 Hepatic toxicity grade 3/4‐subgroup analysis by dose of IDA versus dose of DNR | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 49.1 12 mg/m²/d IDA versus 40 mg/m²/d DNR | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.27, 8.38] |
| 49.2 12 mg/m²/d IDA versus 45 mg/m²/d DNR | 2 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.72, 3.01] |
| 49.3 8 mg/m²/d IDA versus 50 mg/m²/d DNR | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.44, 2.13] |
| 49.4 12 mg/m²/d IDA versus 80 mg/m²/d DNR | 1 | 483 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.32, 0.93] |
| 50 Renal toxicity grade 3/4‐overall analysis | 4 | 743 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.75, 4.00] |
| 51 Renal toxicity grade 3/4‐sensitivity analysis by random‐effects model | 4 | 743 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [0.70, 4.04] |
| 52 Renal toxicity grade 3/4‐subgroup analysis by dose of IDA | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 52.1 8 mg/m²/d | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.14, 6.72] |
| 52.2 12 mg/m²/d | 3 | 523 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.77, 5.05] |
| 53 Renal toxicity grade 3/4‐subgroup analysis by dose of IDA versus dose of DNR | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 53.1 12 mg/m²/d IDA versus 40 mg/m²/d DNR | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.00] |
| 53.2 12 mg/m²/d IDA versus 45 mg/m²/d DNR | 2 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.71, 5.05] |
| 53.3 8 mg/m²/d IDA versus 50 mg/m²/d DNR | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.14, 6.72] |
| 54 Cardiac toxicity grade 3/4‐overall analysis | 6 | 2795 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.70, 1.37] |
| 55 Cardiac toxicity grade 3/4‐sensitivity analysis by random‐effects model | 6 | 2795 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.67, 1.43] |
| 56 Cardiac toxicity grade 3/4‐subgroup analysis by dose of IDA | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 56.1 8 mg/m²/d | 1 | 818 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.61, 1.59] |
| 56.2 12 mg/m²/d | 5 | 1977 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.60, 1.57] |
| 57 Cardiac toxicity grade 3/4‐subgroup analysis by total dose of DNR | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 57.1 < 180 mg/m2 | 3 | 523 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.36, 1.28] |
| 57.2 ≧ 180 mg/m2 | 3 | 2272 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.76, 1.70] |
| 58 Cardiac toxicity grade 3/4‐subgroup analysis by dose of IDA versus dose of DNR | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 58.1 12 mg/m²/d IDA versus 40 mg/m²/d DNR | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.89] |
| 58.2 12 mg/m²/d IDA versus 45 mg/m²/d DNR | 2 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.37, 1.35] |
| 58.3 12 mg/m²/d IDA versus 50 mg/m²/d DNR | 2 | 1121 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.67, 4.05] |
| 58.4 8 mg/m²/d IDA versus 60 mg/m²/d DNR | 1 | 818 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.61, 1.59] |
| 58.5 12 mg/m²/d IDA versus 80 mg/m²/d DNR | 1 | 397 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.29, 3.92] |
| 59 Skin toxicity grade 3/4‐overall analysis | 3 | 761 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.47, 2.23] |
| 60 Skin toxicity grade 3/4‐sensitivity analysis by random‐effects model | 3 | 761 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.41, 2.50] |
| 61 Skin toxicity grade 3/4‐subgroup analysis by total dose of DNR | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 61.1 < 180 mg/m2 | 2 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.45, 6.18] |
| 61.2 ≧ 180 mg/m2 | 1 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.28, 2.07] |
| 62 Skin toxicity grade 3/4‐subgroup analysis by dose of IDA versus dose of DNR | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 62.1 12 mg/m²/d IDA versus 40 mg/m²/d DNR | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.89] |
| 62.2 12 mg/m²/d IDA versus 45 mg/m²/d DNR | 1 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [0.53, 13.57] |
| 62.3 12 mg/m²/d IDA versus 80 mg/m²/d DNR | 1 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.28, 2.07] |
| 63 Central neurotoxicity grade 3/4‐overall analysis | 2 | 707 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.49, 3.35] |
| 64 Central neurotoxicity grade 3/4‐sensitivity analysis by random‐effects model | 2 | 707 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.49, 3.35] |
| 65 Central neurotoxicity grade 3/4‐subgroup analysis by dose of IDA | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 65.1 8 mg/m²/d | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.20, 4.67] |
| 65.2 12 mg/m²/d | 1 | 487 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.45, 5.11] |
| 66 Central neurotoxicity grade 3/4‐subgroup analysis by total dose of DNR | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 66.1 < 180 mg/m2 | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.20, 4.67] |
| 66.2 ≧ 180 mg/m2 | 1 | 487 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.45, 5.11] |
| 67 Central neurotoxicity grade 3/4‐subgroup analysis by dose of IDA versus dose of DNR | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 67.1 8 mg/m²/d IDA versus 50 mg/m²/d DNR | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.20, 4.67] |
| 67.2 12 mg/m²/d IDA versus 80 mg/m²/d DNR | 1 | 487 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.45, 5.11] |
| 68 Bleeding grade 3/4‐overall analysis | 4 | 2299 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.65, 1.45] |
| 69 Bleeding grade 3/4‐sensitivity analysis by random‐effects model | 4 | 2299 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.65, 1.45] |
| 70 Bleeding grade 3/4‐subgroup analysis by dose of IDA | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 70.1 8 mg/m²/d | 1 | 818 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.60, 1.96] |
| 70.2 12 mg/m²/d | 3 | 1481 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.51, 1.53] |
| 71 Bleeding grade 3/4‐subgroup analysis by total dose of DNR | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 71.1 < 180 mg/m2 | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.00] |
| 71.2 ≧ 180 mg/m2 | 3 | 2235 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.63, 1.42] |
| 72 Bleeding grade 3/4‐subgroup analysis by dose of IDA versus dose of DNR | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 72.1 12 mg/m²/d IDA versus 40 mg/m²/d DNR | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.00] |
| 72.2 12 mg/m²/d IDA versus 50 mg/m²/d DNR | 2 | 1121 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.48, 1.54] |
| 72.3 8 mg/m²/d IDA versus 60 mg/m²/d DNR | 1 | 818 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.60, 1.96] |
| 72.4 12 mg/m²/d IDA versus 80 mg/m²/d DNR | 2 | 1417 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.48, 1.48] |
| 73 Stomatitis grade 3/4‐overall analysis | 2 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.78, 3.47] |
| 74 Stomatitis grade 3/4‐sensitivity analysis by random‐effects model | 2 | 284 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.75, 3.38] |
| 75 Stomatitis grade 3/4‐subgroup analysis by dose of IDA versus dose of DNR | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 75.1 12 mg/m²/d IDA versus 40 mg/m²/d DNR | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.47, 33.86] |
| 75.2 8 mg/m²/d IDA versus 50 mg/m²/d DNR | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.62, 3.12] |
| 76 Mucositis grade 3/4‐overall analysis | 5 | 2000 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.04, 1.44] |
| 77 Mucositis grade 3/4‐sensitivity analysis by random‐effects model | 5 | 2000 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.92, 1.61] |
| 78 Mucositis grade 3/4‐subgroup analysis by dose of IDA | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 78.1 8 mg/m²/d | 1 | 818 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.05, 1.60] |
| 78.2 12 mg/m²/d | 4 | 1182 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.87, 1.47] |
| 79 Mucositis grade 3/4‐subgroup analysis by total dose of DNR | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 79.1 < 180 mg/m2 | 2 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.53, 2.44] |
| 79.2 ≧ 180 mg/m2 | 3 | 1662 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [1.04, 1.45] |
| 80 Mucositis grade 3/4‐subgroup analysis by dose of IDA versus dose of DNR | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 80.1 12 mg/m²/d IDA versus 45 mg/m²/d DNR | 1 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.30, 1.91] |
| 80.2 12 mg/m²/d IDA versus 50 mg/m²/d DNR | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.63, 14.27] |
| 80.3 8 mg/m²/d IDA versus 60 mg/m²/d DNR | 1 | 818 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.05, 1.60] |
| 80.4 12 mg/m²/d IDA versus 80 mg/m²/d DNR | 2 | 844 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.86, 1.50] |
| 81 Mucositis grade 3/4‐subgroup analysis by age | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 81.1 < 15 years | 1 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.71, 1.30] |
| 81.2 ≥ 15 years to < 60 years | 2 | 938 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.08, 1.63] |
| 82 Infection grade 3/4‐overall analysis | 5 | 3095 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.99, 1.14] |
| 83 Infection grade 3/4‐sensitivity analysis by random‐effects analysis | 5 | 3095 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.97, 1.11] |
| 84 Infection grade 3/4‐subgroup analysis by dose of IDA | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 84.1 8 mg/m²/d | 1 | 818 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.94, 1.07] |
| 84.2 10 mg/m²/d | 1 | 1438 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.94, 1.28] |
| 84.3 12 mg/m²/d | 3 | 839 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.95, 1.47] |
| 85 Infection grade 3/4‐subgroup analysis by total dose of DNR | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 85.1 < 180 mg/m2 | 3 | 1790 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.96, 1.29] |
| 85.2 ≧ 180 mg/m2 | 2 | 1305 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.97, 1.11] |
| 86 Infection grade 3/4‐subgroup analysis by dose of IDA versus dose of DNR | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 86.1 12 mg/m²/d IDA versus 40 mg/m²/d DNR | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.43, 2.34] |
| 86.2 10 mg/m²/d IDA versus 50 mg/m²/d DNR | 1 | 1438 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.94, 1.28] |
| 86.3 8 mg/m²/d IDA versus 60 mg/m²/d DNR | 1 | 818 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.94, 1.07] |
| 86.4 12 mg/m²/d IDA versus 60 mg/m²/d DNR | 1 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.78, 2.10] |
| 86.5 12 mg/m²/d IDA versus 80 mg/m²/d DNR | 1 | 487 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.91, 1.51] |
| 87 Infection grade 3/4‐subgroup analysis by age | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 87.1 < 15 years | 1 | 487 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.91, 1.51] |
| 87.2 ≥ 15 years to < 60 years | 2 | 2256 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.97, 1.12] |
| 88 Sepsis grade 3/4‐overall analysis | 2 | 1417 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.96, 1.77] |
| 89 Sepsis grade 3/4‐sensitivity analysis by random‐effects model | 2 | 1417 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.74, 2.28] |
| 90 Sepsis grade 3/4‐subgroup analysis by dose of IDA versus dose of DNR | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 90.1 12 mg/m²/d IDA versus 50 mg/m²/d DNR | 1 | 1057 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.10, 2.78] |
| 90.2 12 mg/m²/d IDA versus 80 mg/m²/d DNR | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.66, 1.47] |
1.1. Analysis.
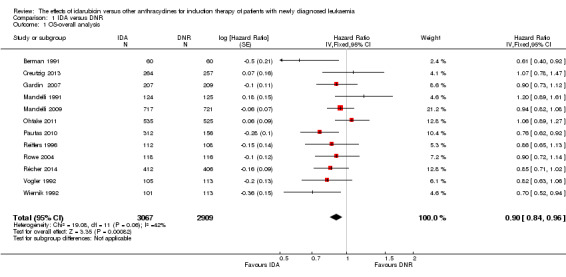
Comparison 1 IDA versus DNR, Outcome 1 OS‐overall analysis.
1.2. Analysis.
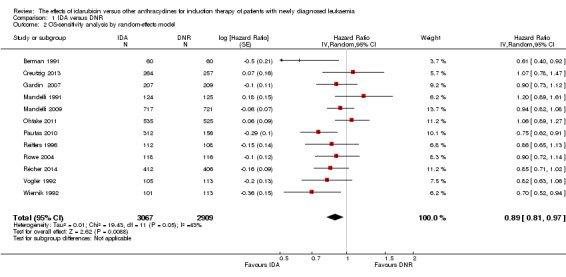
Comparison 1 IDA versus DNR, Outcome 2 OS‐sensitivity analysis by random‐effects model.
1.3. Analysis.
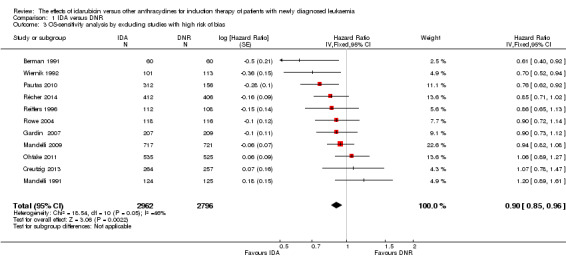
Comparison 1 IDA versus DNR, Outcome 3 OS‐sensitivity analysis by excluding studies with high risk of bias.
1.4. Analysis.
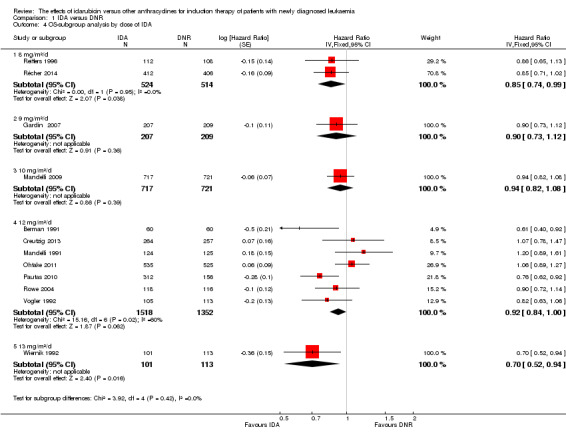
Comparison 1 IDA versus DNR, Outcome 4 OS‐subgroup analysis by dose of IDA.
1.5. Analysis.
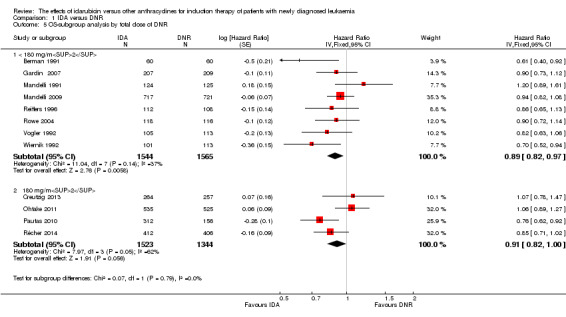
Comparison 1 IDA versus DNR, Outcome 5 OS‐subgroup analysis by total dose of DNR.
1.6. Analysis.
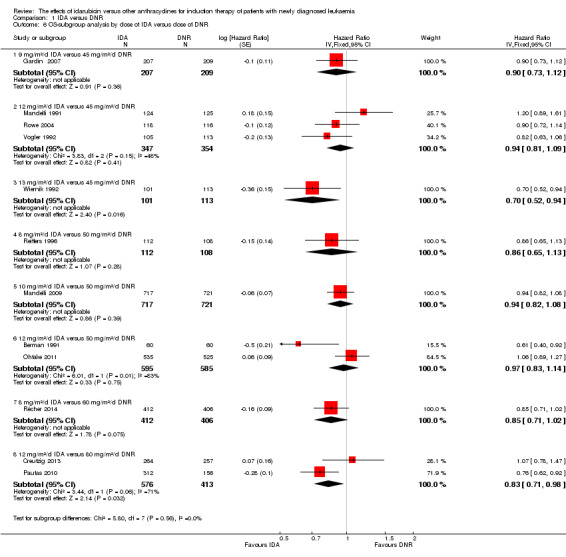
Comparison 1 IDA versus DNR, Outcome 6 OS‐subgroup analysis by dose of IDA versus dose of DNR.
1.7. Analysis.
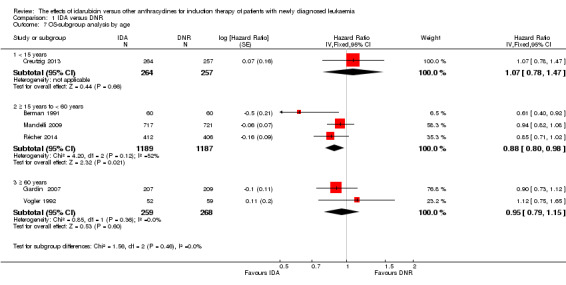
Comparison 1 IDA versus DNR, Outcome 7 OS‐subgroup analysis by age.
1.8. Analysis.
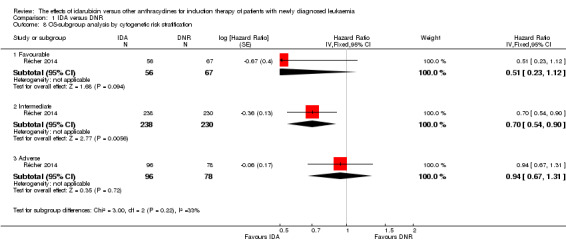
Comparison 1 IDA versus DNR, Outcome 8 OS‐subgroup analysis by cytogenetic risk stratification.
1.9. Analysis.
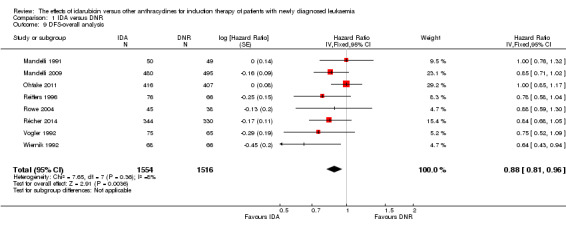
Comparison 1 IDA versus DNR, Outcome 9 DFS‐overall analysis.
1.10. Analysis.
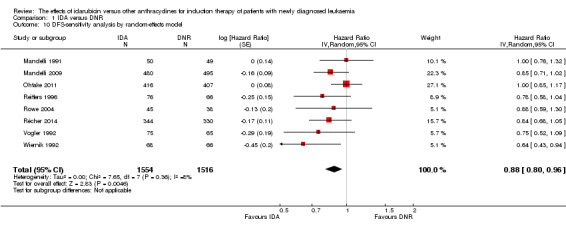
Comparison 1 IDA versus DNR, Outcome 10 DFS‐sensitivity analysis by random‐effects model.
1.11. Analysis.
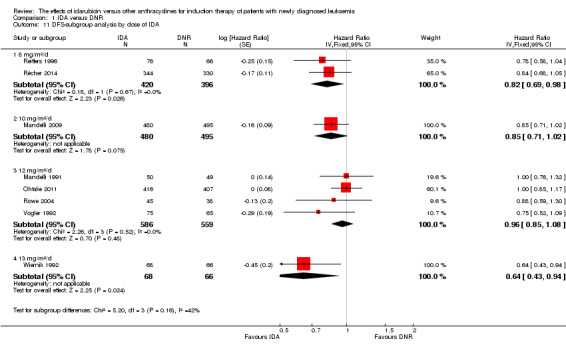
Comparison 1 IDA versus DNR, Outcome 11 DFS‐subgroup analysis by dose of IDA.
1.12. Analysis.
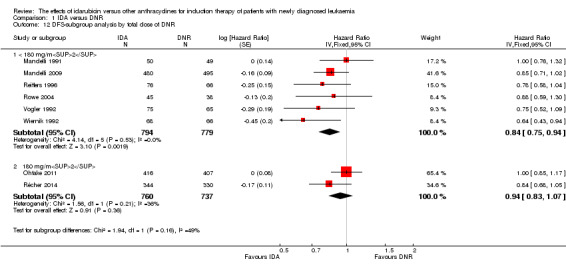
Comparison 1 IDA versus DNR, Outcome 12 DFS‐subgroup analysis by total dose of DNR.
1.13. Analysis.
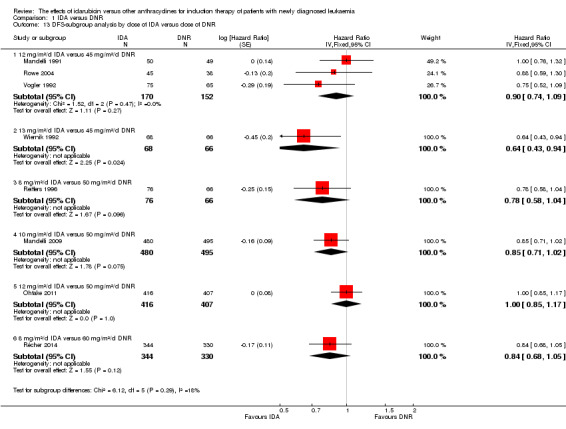
Comparison 1 IDA versus DNR, Outcome 13 DFS‐subgroup analysis by dose of IDA versus dose of DNR.
1.14. Analysis.
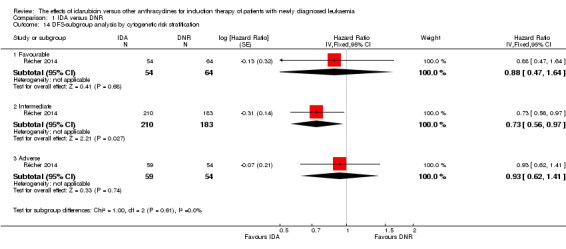
Comparison 1 IDA versus DNR, Outcome 14 DFS‐subgroup analysis by cytogenetic risk stratification.
1.15. Analysis.
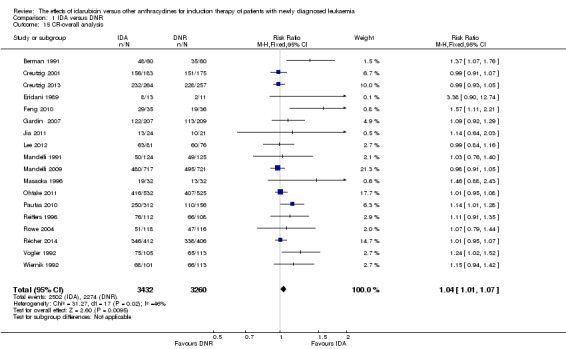
Comparison 1 IDA versus DNR, Outcome 15 CR‐overall analysis.
1.16. Analysis.
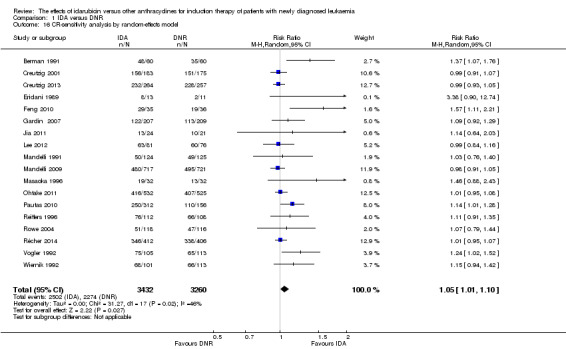
Comparison 1 IDA versus DNR, Outcome 16 CR‐sensitivity analysis by random‐effects model.
1.17. Analysis.
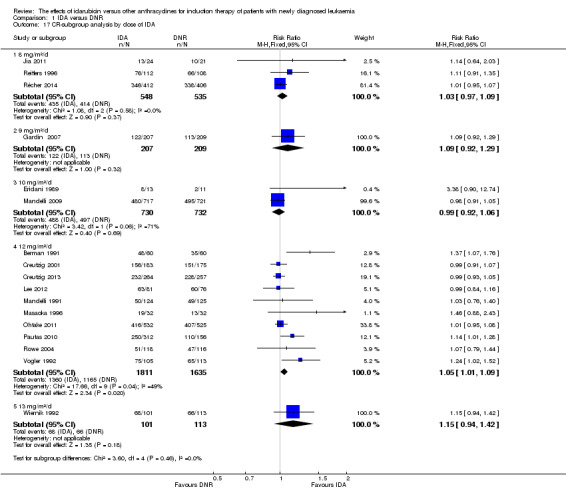
Comparison 1 IDA versus DNR, Outcome 17 CR‐subgroup analysis by dose of IDA.
1.18. Analysis.
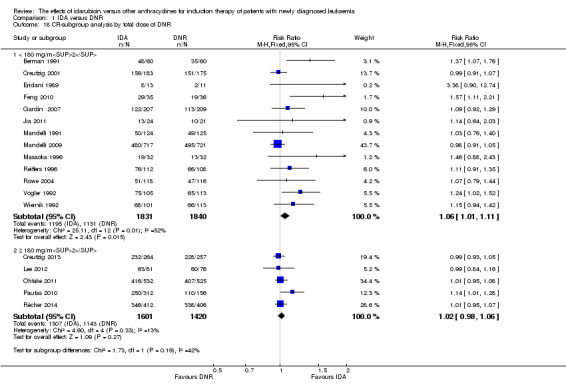
Comparison 1 IDA versus DNR, Outcome 18 CR‐subgroup analysis by total dose of DNR.
1.19. Analysis.
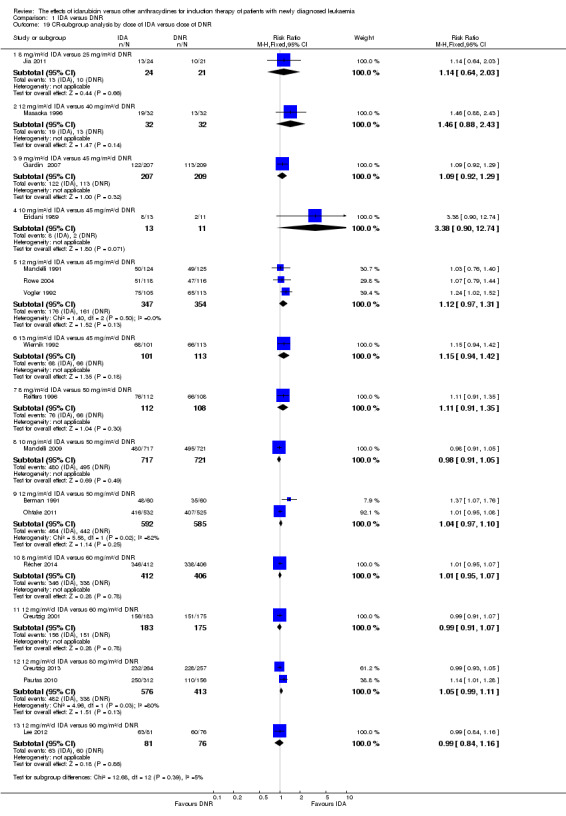
Comparison 1 IDA versus DNR, Outcome 19 CR‐subgroup analysis by dose of IDA versus dose of DNR.
1.20. Analysis.
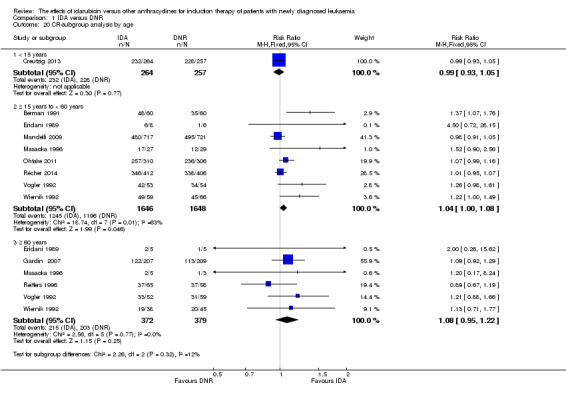
Comparison 1 IDA versus DNR, Outcome 20 CR‐subgroup analysis by age.
1.21. Analysis.
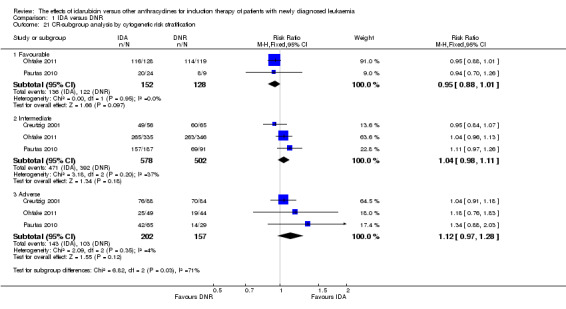
Comparison 1 IDA versus DNR, Outcome 21 CR‐subgroup analysis by cytogenetic risk stratification.
1.22. Analysis.
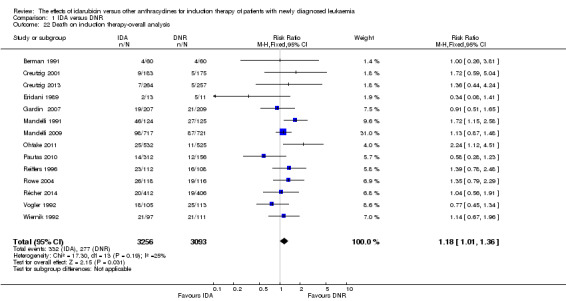
Comparison 1 IDA versus DNR, Outcome 22 Death on induction therapy‐overall analysis.
1.23. Analysis.
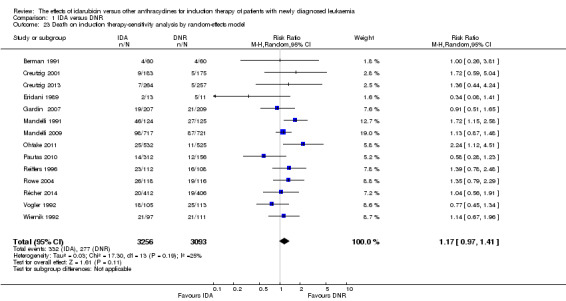
Comparison 1 IDA versus DNR, Outcome 23 Death on induction therapy‐sensitivity analysis by random‐effects model.
1.24. Analysis.
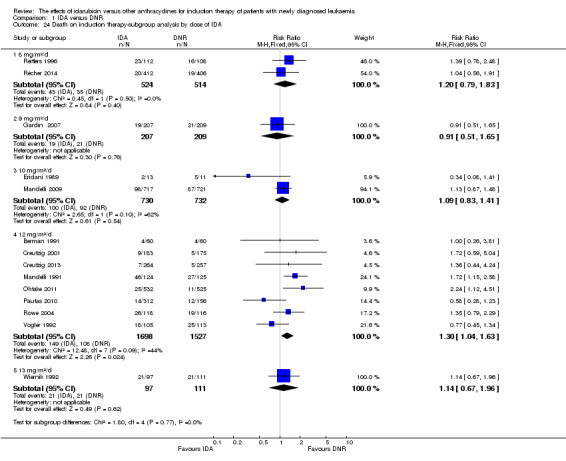
Comparison 1 IDA versus DNR, Outcome 24 Death on induction therapy‐subgroup analysis by dose of IDA.
1.25. Analysis.
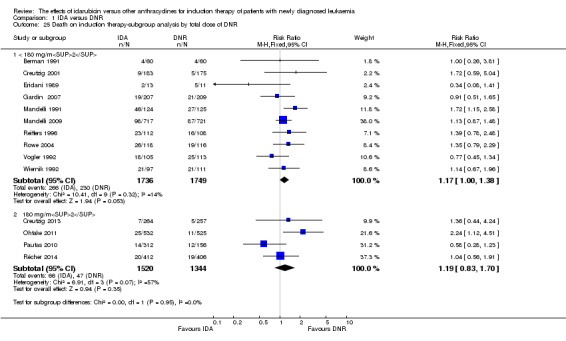
Comparison 1 IDA versus DNR, Outcome 25 Death on induction therapy‐subgroup analysis by total dose of DNR.
1.26. Analysis.
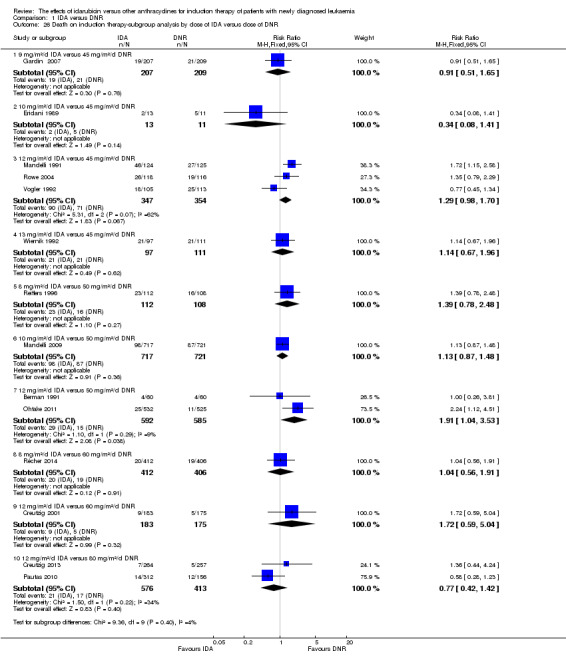
Comparison 1 IDA versus DNR, Outcome 26 Death on induction therapy‐subgroup analysis by dose of IDA versus dose of DNR.
1.27. Analysis.
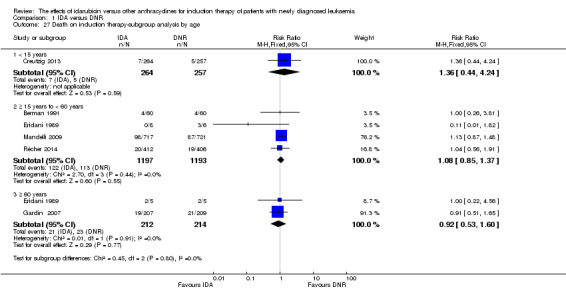
Comparison 1 IDA versus DNR, Outcome 27 Death on induction therapy‐subgroup analysis by age.
1.28. Analysis.
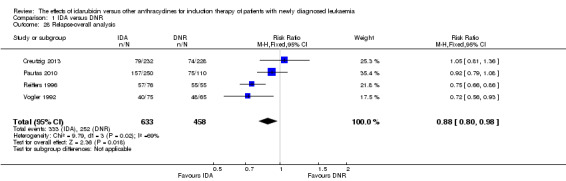
Comparison 1 IDA versus DNR, Outcome 28 Relapse‐overall analysis.
1.29. Analysis.
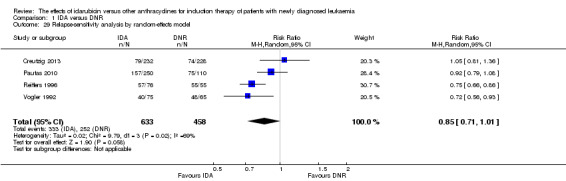
Comparison 1 IDA versus DNR, Outcome 29 Relapse‐sensitivity analysis by random‐effects model.
1.30. Analysis.
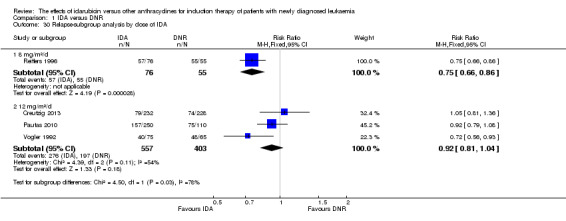
Comparison 1 IDA versus DNR, Outcome 30 Relapse‐subgroup analysis by dose of IDA.
1.31. Analysis.
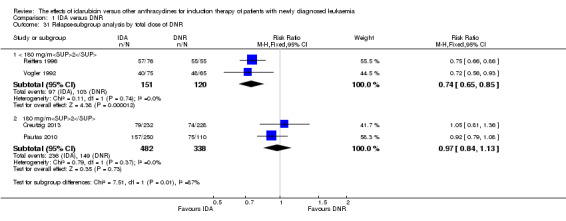
Comparison 1 IDA versus DNR, Outcome 31 Relapse‐subgroup analysis by total dose of DNR.
1.32. Analysis.
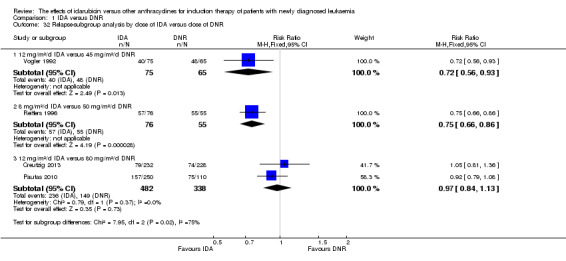
Comparison 1 IDA versus DNR, Outcome 32 Relapse‐subgroup analysis by dose of IDA versus dose of DNR.
1.33. Analysis.
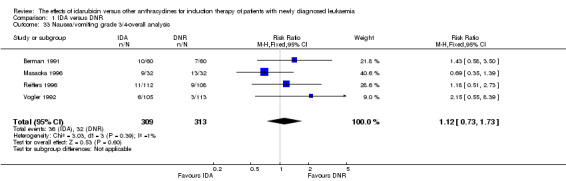
Comparison 1 IDA versus DNR, Outcome 33 Nausea/vomiting grade 3/4‐overall analysis.
1.34. Analysis.
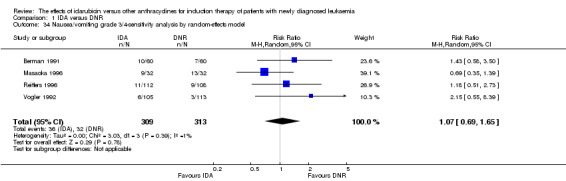
Comparison 1 IDA versus DNR, Outcome 34 Nausea/vomiting grade 3/4‐sensitivity analysis by random‐effects model.
1.35. Analysis.
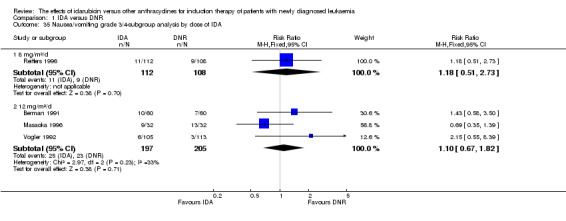
Comparison 1 IDA versus DNR, Outcome 35 Nausea/vomiting grade 3/4‐subgroup analysis by dose of IDA.
1.36. Analysis.
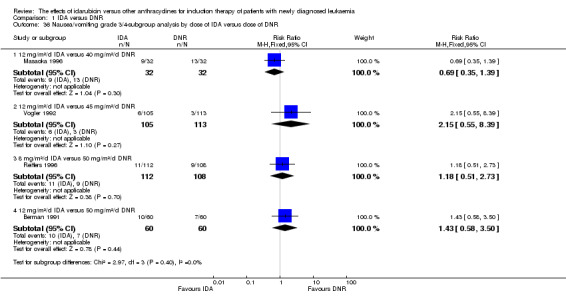
Comparison 1 IDA versus DNR, Outcome 36 Nausea/vomiting grade 3/4‐subgroup analysis by dose of IDA versus dose of DNR.
1.37. Analysis.
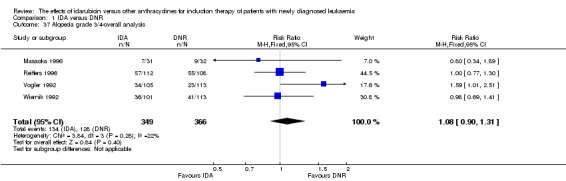
Comparison 1 IDA versus DNR, Outcome 37 Alopecia grade 3/4‐overall analysis.
1.38. Analysis.
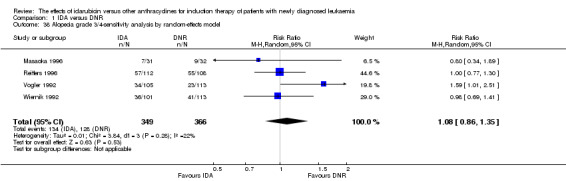
Comparison 1 IDA versus DNR, Outcome 38 Alopecia grade 3/4‐sensitivity analysis by random‐effects model.
1.39. Analysis.
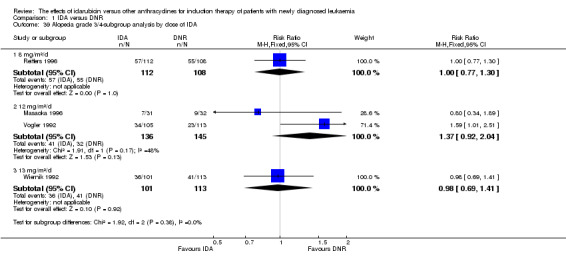
Comparison 1 IDA versus DNR, Outcome 39 Alopecia grade 3/4‐subgroup analysis by dose of IDA.
1.40. Analysis.
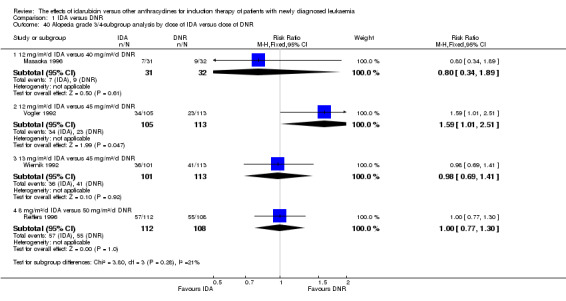
Comparison 1 IDA versus DNR, Outcome 40 Alopecia grade 3/4‐subgroup analysis by dose of IDA versus dose of DNR.
1.41. Analysis.
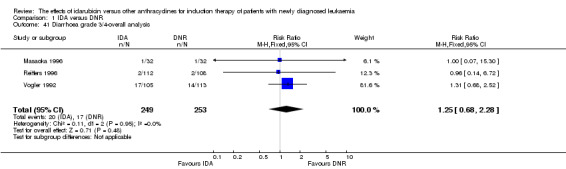
Comparison 1 IDA versus DNR, Outcome 41 Diarrhoea grade 3/4‐overall analysis.
1.42. Analysis.
Comparison 1 IDA versus DNR, Outcome 42 Diarrhoea grade 3/4‐sensitivity analysis by random‐effects model.
1.43. Analysis.
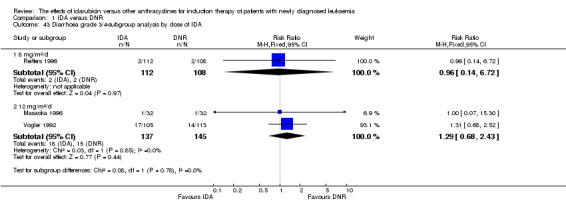
Comparison 1 IDA versus DNR, Outcome 43 Diarrhoea grade 3/4‐subgroup analysis by dose of IDA.
1.44. Analysis.
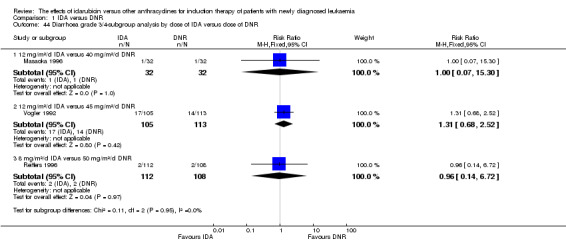
Comparison 1 IDA versus DNR, Outcome 44 Diarrhoea grade 3/4‐subgroup analysis by dose of IDA versus dose of DNR.
1.45. Analysis.
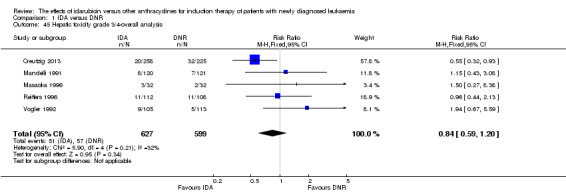
Comparison 1 IDA versus DNR, Outcome 45 Hepatic toxicity grade 3/4‐overall analysis.
1.46. Analysis.
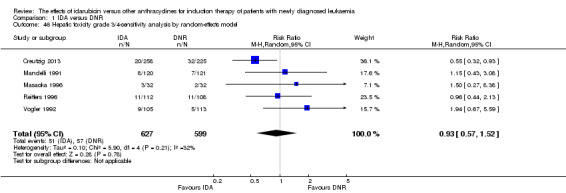
Comparison 1 IDA versus DNR, Outcome 46 Hepatic toxicity grade 3/4‐sensitivity analysis by random‐effects model.
1.47. Analysis.
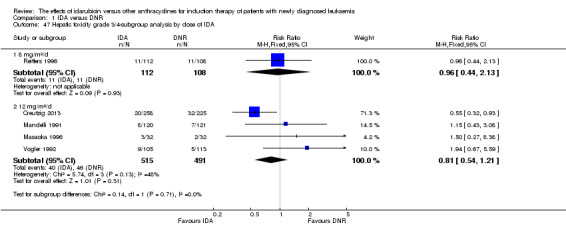
Comparison 1 IDA versus DNR, Outcome 47 Hepatic toxicity grade 3/4‐subgroup analysis by dose of IDA.
1.48. Analysis.
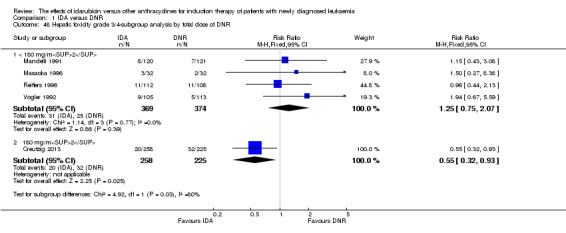
Comparison 1 IDA versus DNR, Outcome 48 Hepatic toxicity grade 3/4‐subgroup analysis by total dose of DNR.
1.49. Analysis.
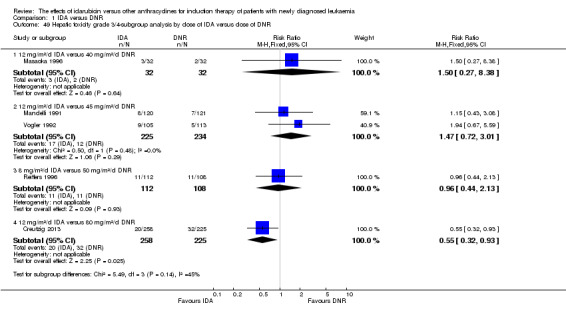
Comparison 1 IDA versus DNR, Outcome 49 Hepatic toxicity grade 3/4‐subgroup analysis by dose of IDA versus dose of DNR.
1.50. Analysis.
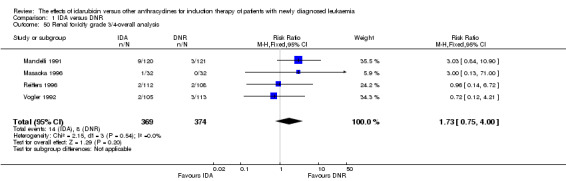
Comparison 1 IDA versus DNR, Outcome 50 Renal toxicity grade 3/4‐overall analysis.
1.51. Analysis.
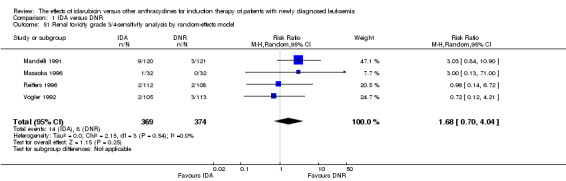
Comparison 1 IDA versus DNR, Outcome 51 Renal toxicity grade 3/4‐sensitivity analysis by random‐effects model.
1.52. Analysis.
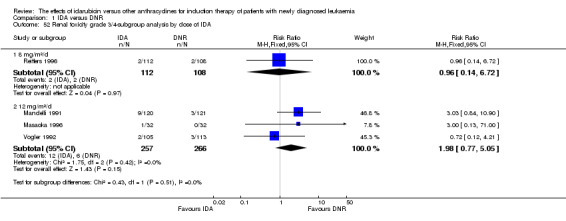
Comparison 1 IDA versus DNR, Outcome 52 Renal toxicity grade 3/4‐subgroup analysis by dose of IDA.
1.53. Analysis.
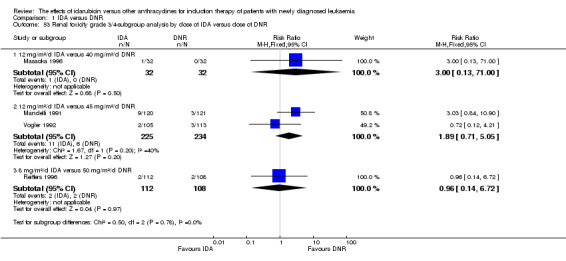
Comparison 1 IDA versus DNR, Outcome 53 Renal toxicity grade 3/4‐subgroup analysis by dose of IDA versus dose of DNR.
1.54. Analysis.
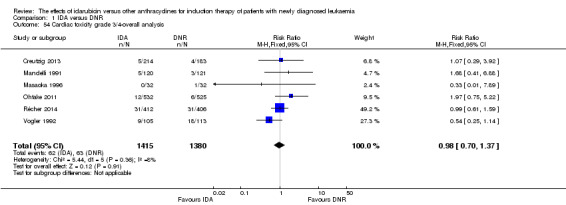
Comparison 1 IDA versus DNR, Outcome 54 Cardiac toxicity grade 3/4‐overall analysis.
1.55. Analysis.
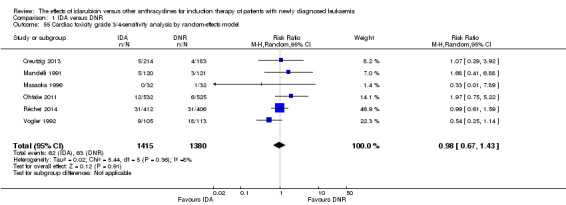
Comparison 1 IDA versus DNR, Outcome 55 Cardiac toxicity grade 3/4‐sensitivity analysis by random‐effects model.
1.56. Analysis.
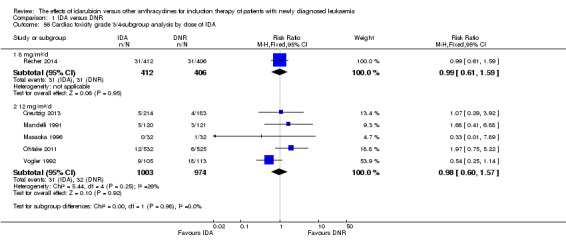
Comparison 1 IDA versus DNR, Outcome 56 Cardiac toxicity grade 3/4‐subgroup analysis by dose of IDA.
1.57. Analysis.
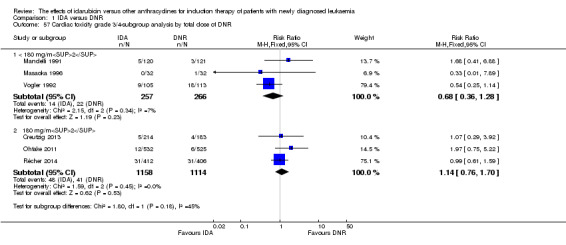
Comparison 1 IDA versus DNR, Outcome 57 Cardiac toxicity grade 3/4‐subgroup analysis by total dose of DNR.
1.58. Analysis.
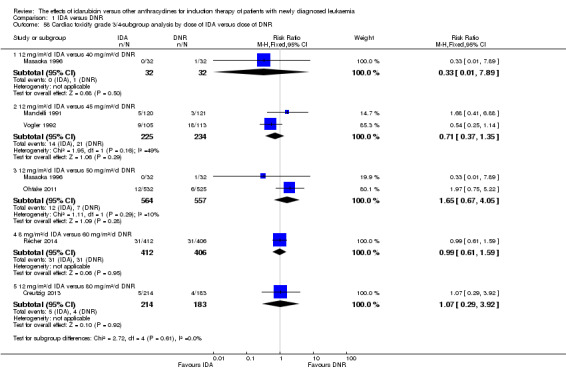
Comparison 1 IDA versus DNR, Outcome 58 Cardiac toxicity grade 3/4‐subgroup analysis by dose of IDA versus dose of DNR.
1.59. Analysis.
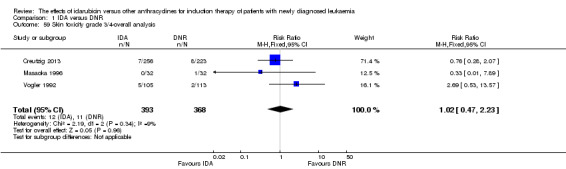
Comparison 1 IDA versus DNR, Outcome 59 Skin toxicity grade 3/4‐overall analysis.
1.60. Analysis.
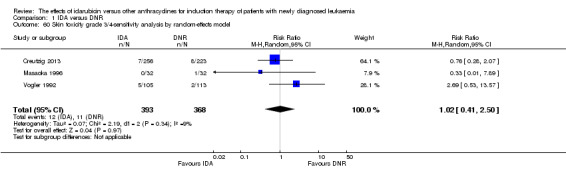
Comparison 1 IDA versus DNR, Outcome 60 Skin toxicity grade 3/4‐sensitivity analysis by random‐effects model.
1.61. Analysis.
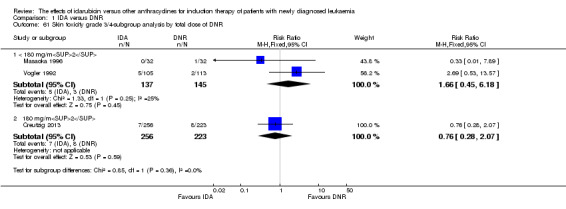
Comparison 1 IDA versus DNR, Outcome 61 Skin toxicity grade 3/4‐subgroup analysis by total dose of DNR.
1.62. Analysis.
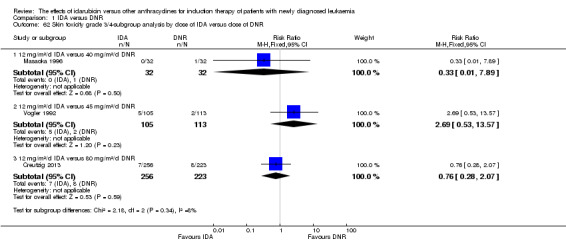
Comparison 1 IDA versus DNR, Outcome 62 Skin toxicity grade 3/4‐subgroup analysis by dose of IDA versus dose of DNR.
1.63. Analysis.
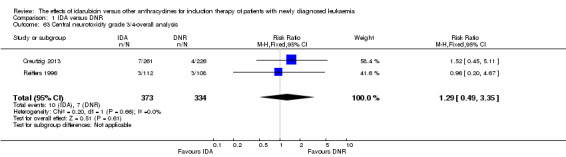
Comparison 1 IDA versus DNR, Outcome 63 Central neurotoxicity grade 3/4‐overall analysis.
1.64. Analysis.
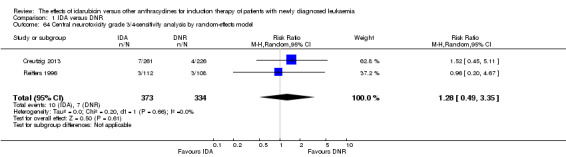
Comparison 1 IDA versus DNR, Outcome 64 Central neurotoxicity grade 3/4‐sensitivity analysis by random‐effects model.
1.65. Analysis.
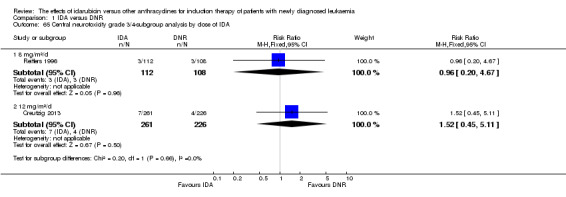
Comparison 1 IDA versus DNR, Outcome 65 Central neurotoxicity grade 3/4‐subgroup analysis by dose of IDA.
1.66. Analysis.

Comparison 1 IDA versus DNR, Outcome 66 Central neurotoxicity grade 3/4‐subgroup analysis by total dose of DNR.
1.67. Analysis.

Comparison 1 IDA versus DNR, Outcome 67 Central neurotoxicity grade 3/4‐subgroup analysis by dose of IDA versus dose of DNR.
1.68. Analysis.
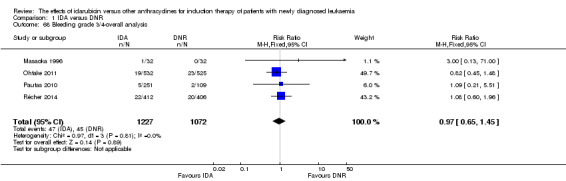
Comparison 1 IDA versus DNR, Outcome 68 Bleeding grade 3/4‐overall analysis.
1.69. Analysis.
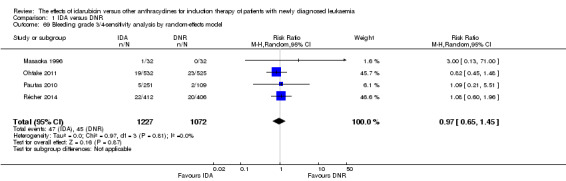
Comparison 1 IDA versus DNR, Outcome 69 Bleeding grade 3/4‐sensitivity analysis by random‐effects model.
1.70. Analysis.
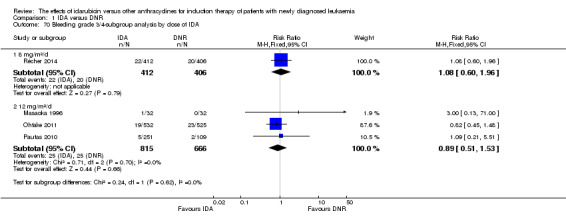
Comparison 1 IDA versus DNR, Outcome 70 Bleeding grade 3/4‐subgroup analysis by dose of IDA.
1.71. Analysis.
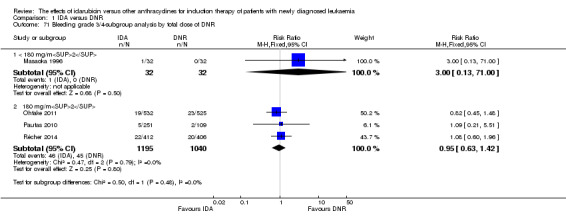
Comparison 1 IDA versus DNR, Outcome 71 Bleeding grade 3/4‐subgroup analysis by total dose of DNR.
1.72. Analysis.
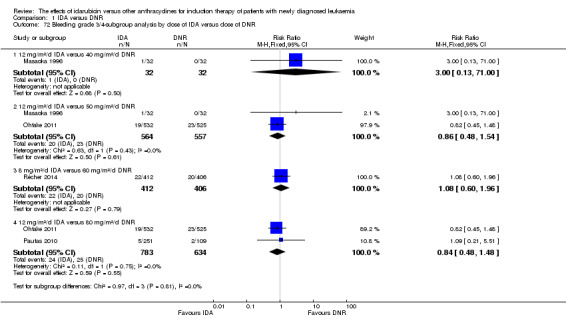
Comparison 1 IDA versus DNR, Outcome 72 Bleeding grade 3/4‐subgroup analysis by dose of IDA versus dose of DNR.
1.73. Analysis.
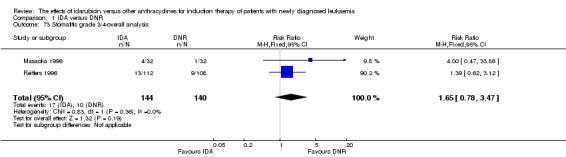
Comparison 1 IDA versus DNR, Outcome 73 Stomatitis grade 3/4‐overall analysis.
1.74. Analysis.
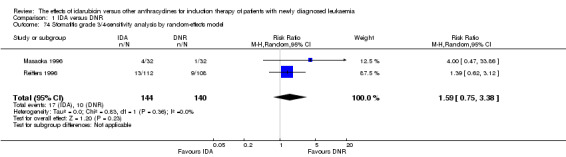
Comparison 1 IDA versus DNR, Outcome 74 Stomatitis grade 3/4‐sensitivity analysis by random‐effects model.
1.75. Analysis.
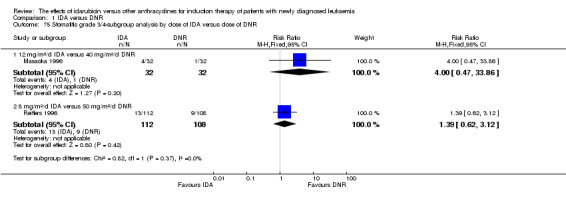
Comparison 1 IDA versus DNR, Outcome 75 Stomatitis grade 3/4‐subgroup analysis by dose of IDA versus dose of DNR.
1.76. Analysis.
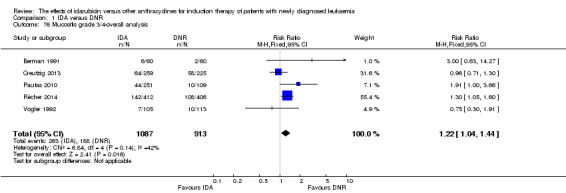
Comparison 1 IDA versus DNR, Outcome 76 Mucositis grade 3/4‐overall analysis.
1.77. Analysis.
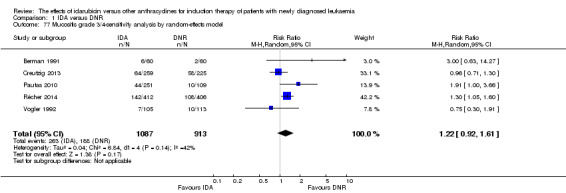
Comparison 1 IDA versus DNR, Outcome 77 Mucositis grade 3/4‐sensitivity analysis by random‐effects model.
1.78. Analysis.
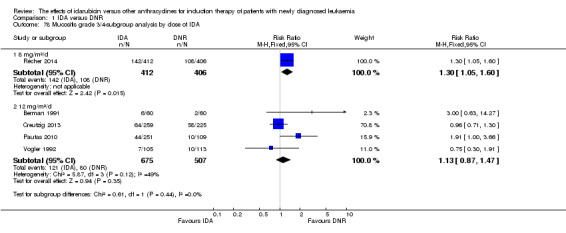
Comparison 1 IDA versus DNR, Outcome 78 Mucositis grade 3/4‐subgroup analysis by dose of IDA.
1.79. Analysis.
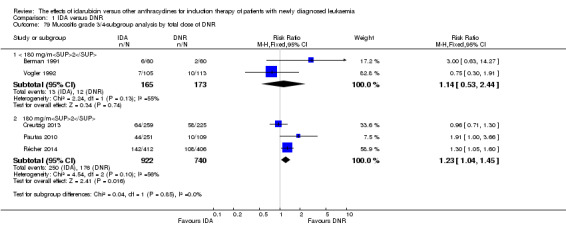
Comparison 1 IDA versus DNR, Outcome 79 Mucositis grade 3/4‐subgroup analysis by total dose of DNR.
1.80. Analysis.
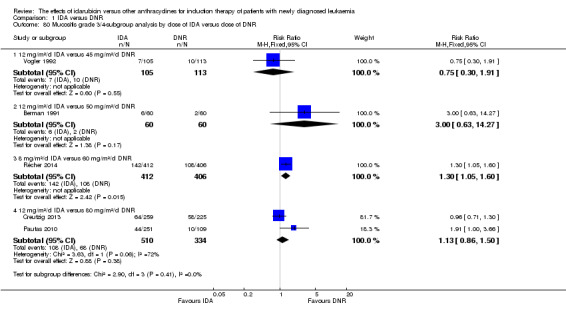
Comparison 1 IDA versus DNR, Outcome 80 Mucositis grade 3/4‐subgroup analysis by dose of IDA versus dose of DNR.
1.81. Analysis.
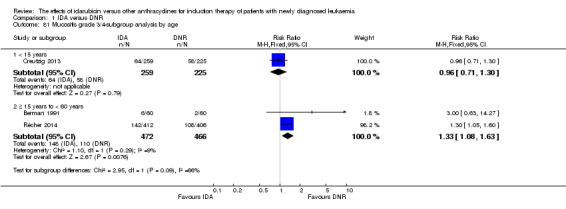
Comparison 1 IDA versus DNR, Outcome 81 Mucositis grade 3/4‐subgroup analysis by age.
1.82. Analysis.
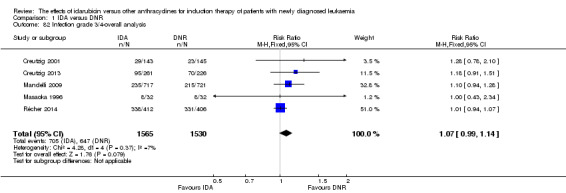
Comparison 1 IDA versus DNR, Outcome 82 Infection grade 3/4‐overall analysis.
1.83. Analysis.
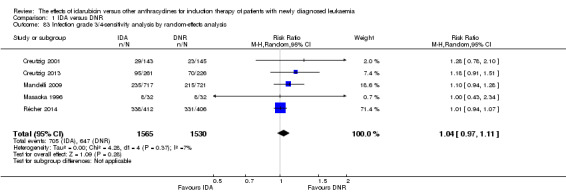
Comparison 1 IDA versus DNR, Outcome 83 Infection grade 3/4‐sensitivity analysis by random‐effects analysis.
1.84. Analysis.
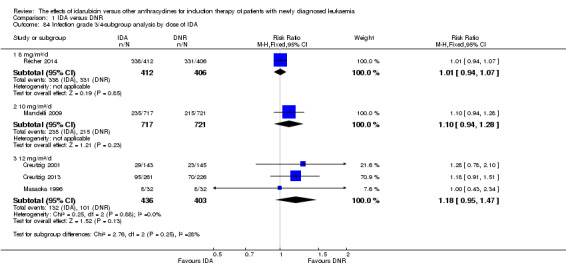
Comparison 1 IDA versus DNR, Outcome 84 Infection grade 3/4‐subgroup analysis by dose of IDA.
1.85. Analysis.
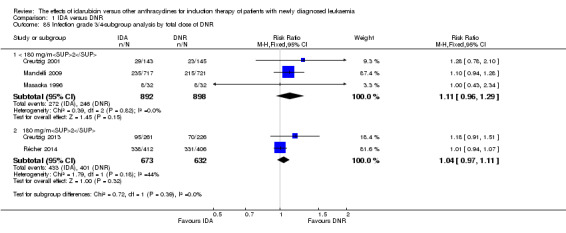
Comparison 1 IDA versus DNR, Outcome 85 Infection grade 3/4‐subgroup analysis by total dose of DNR.
1.86. Analysis.
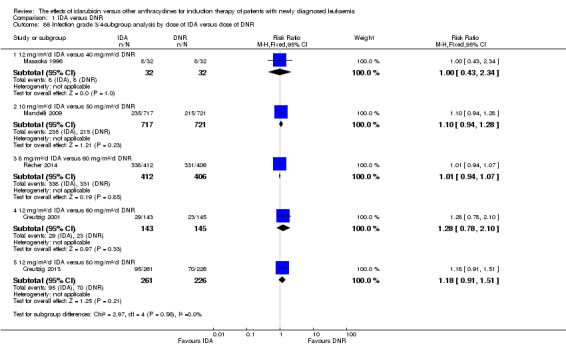
Comparison 1 IDA versus DNR, Outcome 86 Infection grade 3/4‐subgroup analysis by dose of IDA versus dose of DNR.
1.87. Analysis.
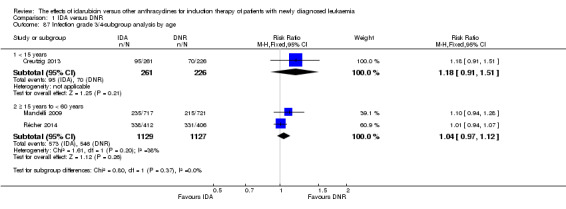
Comparison 1 IDA versus DNR, Outcome 87 Infection grade 3/4‐subgroup analysis by age.
1.88. Analysis.
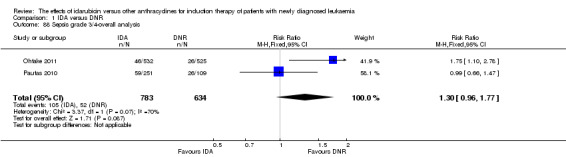
Comparison 1 IDA versus DNR, Outcome 88 Sepsis grade 3/4‐overall analysis.
1.89. Analysis.
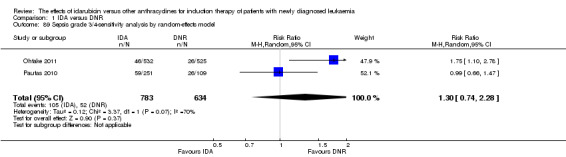
Comparison 1 IDA versus DNR, Outcome 89 Sepsis grade 3/4‐sensitivity analysis by random‐effects model.
1.90. Analysis.

Comparison 1 IDA versus DNR, Outcome 90 Sepsis grade 3/4‐subgroup analysis by dose of IDA versus dose of DNR.
Comparison 2. IDA versus MIT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 OS‐overall analysis | 6 | 2171 | Hazard Ratio (Fixed, 95% CI) | 0.98 [0.89, 1.08] |
| 2 OS‐sensitivity analysis by random‐effects model | 6 | 2171 | Hazard Ratio (Random, 95% CI) | 0.97 [0.83, 1.12] |
| 3 OS‐sensitivity analysis by excluding studies with high risk of bias | 5 | 2115 | Hazard Ratio (Fixed, 95% CI) | 1.01 [0.92, 1.11] |
| 4 OS‐subgroup analysis by dose of IDA | 6 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 4.1 8 mg/m²/d | 2 | 220 | Hazard Ratio (Fixed, 95% CI) | 1.06 [0.81, 1.39] |
| 4.2 10 mg/m²/d | 2 | 1663 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.89, 1.11] |
| 4.3 12 mg/m²/d | 2 | 288 | Hazard Ratio (Fixed, 95% CI) | 0.90 [0.73, 1.11] |
| 5 OS‐subgroup analysis by age | 2 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 5.1 ≥ 15 years to < 60 years | 1 | 1436 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.88, 1.11] |
| 5.2 ≥ 60 years | 1 | 160 | Hazard Ratio (Fixed, 95% CI) | 1.03 [0.75, 1.41] |
| 6 DFS‐overall analysis | 4 | 249 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.70, 1.10] |
| 7 DFS‐sensitivity analysis by random‐effects model | 4 | 249 | Hazard Ratio (Random, 95% CI) | 0.86 [0.62, 1.21] |
| 8 DFS‐subgroup analysis by dose of IDA | 4 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 8.1 8 mg/m²/d | 2 | 123 | Hazard Ratio (Fixed, 95% CI) | 1.06 [0.75, 1.50] |
| 8.2 12 mg/m²/d | 2 | 126 | Hazard Ratio (Fixed, 95% CI) | 0.75 [0.55, 1.02] |
| 9 CR‐overall analysis | 8 | 2411 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.92, 1.03] |
| 10 CR‐sensitivity analysis by random‐effects model | 8 | 2411 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.92, 1.02] |
| 11 CR‐subgroup analysis by dose of IDA | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 8 mg/m²/d | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.70, 1.11] |
| 11.2 10 mg/m²/d | 2 | 1663 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.90, 1.02] |
| 11.3 12 mg/m²/d | 2 | 295 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.80, 1.24] |
| 12 CR‐subgroup analysis by age | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 ≥ 15 years to < 60 years | 2 | 1490 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.90, 1.03] |
| 12.2 ≥ 60 years | 2 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.71, 1.18] |
| 13 Death on induction therapy‐overall analysis | 5 | 2055 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.88, 1.38] |
| 14 Death on induction therapy‐sensitivity analysis by random‐effects model | 5 | 2055 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.88, 1.38] |
| 15 Death on induction therapy‐subgroup analysis by dose of IDA | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 8 mg/m²/d | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.33, 1.92] |
| 15.2 10 mg/m²/d | 1 | 1436 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.81, 1.38] |
| 15.3 12 mg/m²/d | 2 | 295 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.90, 2.43] |
| 16 Death on induction therapy‐subgroup analysis by age | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 ≥ 15 years to < 60 years | 1 | 1436 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.81, 1.38] |
| 16.2 ≥ 60 years | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.33, 1.92] |
| 17 Relapse‐overall analysis | 3 | 328 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.80, 1.22] |
| 18 Relapse‐sensitivity analysis by random‐effects model | 3 | 328 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.80, 1.19] |
| 19 Relapse‐subgroup analysis by dose of IDA | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 8 mg/m²/d | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.75, 1.53] |
| 19.2 10 mg/m²/d | 1 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.68, 1.37] |
| 19.3 12 mg/m²/d | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.66, 1.26] |
| 20 Nausea/vomiting grade 3/4‐overall analysis | 3 | 387 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.66, 1.61] |
| 21 Nausea/vomiting grade 3/4‐sensitivity analysis by random‐effects model | 3 | 387 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.67, 1.63] |
| 22 Nausea/vomiting grade 3/4‐subgroup analysis by dose of IDA | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.1 8 mg/m²/d | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.22, 2.87] |
| 22.2 12 mg/m²/d | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.13, 5.68] |
| 23 Diarrhoea grade 3/4‐overall analysis | 2 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.68, 2.89] |
| 24 Diarrhoea grade 3/4‐sensitivity analysis by random‐effects model | 2 | 223 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.68, 2.88] |
| 25 Diarrhoea grade 3/4‐subgroup analysis by dose of IDA | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 25.1 8 mg/m²/d | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.60, 2.99] |
| 25.2 12 mg/m²/d | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.34, 8.65] |
| 26 Hepatic toxicity grade 3/4‐overall analysis | 2 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.47, 3.17] |
| 27 Hepatic toxicity grade 3/4‐sensitivity analysis by random‐effects analysis | 2 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.47, 3.16] |
| 28 Renal toxicity grade 3/4‐overall analysis | 2 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.03, 2.91] |
| 29 Renal toxicity grade 3/4‐sensitivity analysis by random effects model | 2 | 223 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.03, 2.91] |
| 30 Renal toxicity grade 3/4‐subgroup analysis by dose of IDA | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 30.1 8 mg/m²/d | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.06] |
| 30.2 12 mg/m²/d | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.76] |
| 31 Mucositis grade 3/4‐overall analysis | 2 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.36, 9.92] |
| 32 Mucositis grade 3/4‐sensitivity analysis by random‐effects model | 2 | 223 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.32, 10.21] |
| 33 Mucositis grade 3/4‐subgroup analysis by dose of IDA | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 33.1 8 mg/m²/d | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.32, 28.23] |
| 33.2 12 mg/m²/d | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.06, 13.04] |
| 34 Infection grade 3/4‐overall analysis | 2 | 1663 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.19] |
| 35 Infection grade 3/4‐sensitivity analysis by random‐effects analysis | 2 | 1663 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.78, 1.72] |
2.1. Analysis.
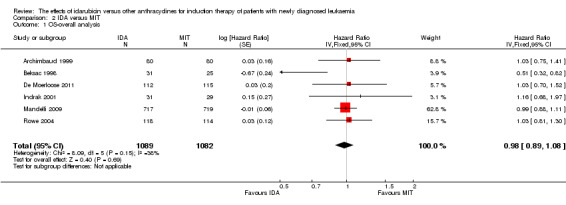
Comparison 2 IDA versus MIT, Outcome 1 OS‐overall analysis.
2.2. Analysis.
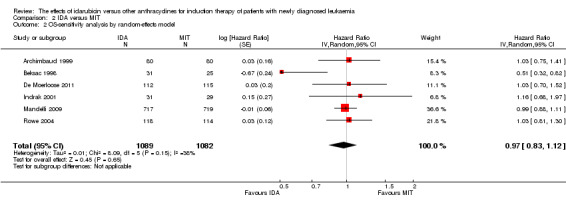
Comparison 2 IDA versus MIT, Outcome 2 OS‐sensitivity analysis by random‐effects model.
2.3. Analysis.
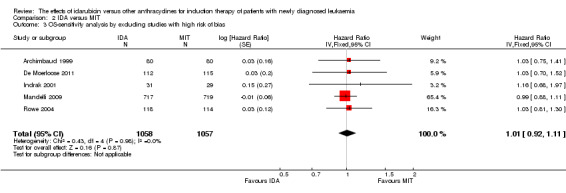
Comparison 2 IDA versus MIT, Outcome 3 OS‐sensitivity analysis by excluding studies with high risk of bias.
2.4. Analysis.
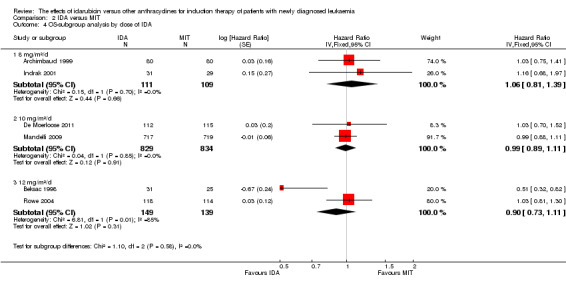
Comparison 2 IDA versus MIT, Outcome 4 OS‐subgroup analysis by dose of IDA.
2.5. Analysis.
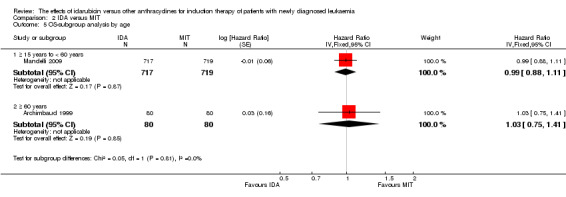
Comparison 2 IDA versus MIT, Outcome 5 OS‐subgroup analysis by age.
2.6. Analysis.
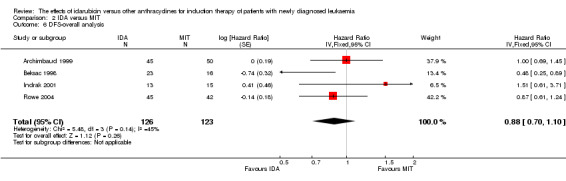
Comparison 2 IDA versus MIT, Outcome 6 DFS‐overall analysis.
2.7. Analysis.
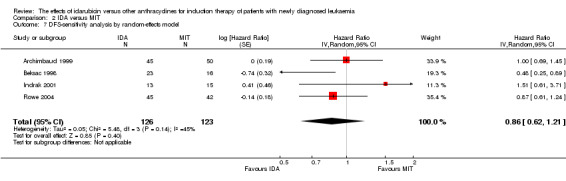
Comparison 2 IDA versus MIT, Outcome 7 DFS‐sensitivity analysis by random‐effects model.
2.8. Analysis.
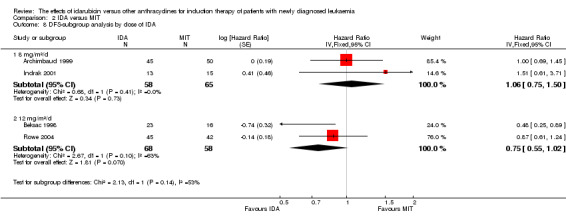
Comparison 2 IDA versus MIT, Outcome 8 DFS‐subgroup analysis by dose of IDA.
2.9. Analysis.
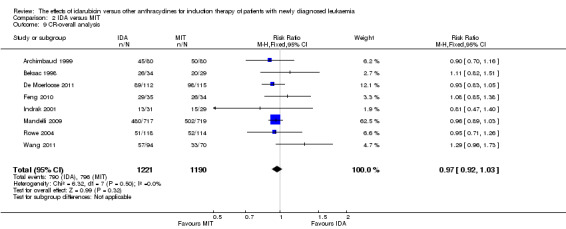
Comparison 2 IDA versus MIT, Outcome 9 CR‐overall analysis.
2.10. Analysis.
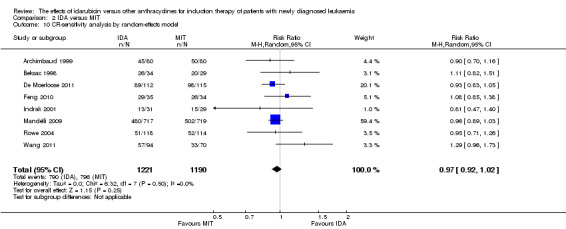
Comparison 2 IDA versus MIT, Outcome 10 CR‐sensitivity analysis by random‐effects model.
2.11. Analysis.
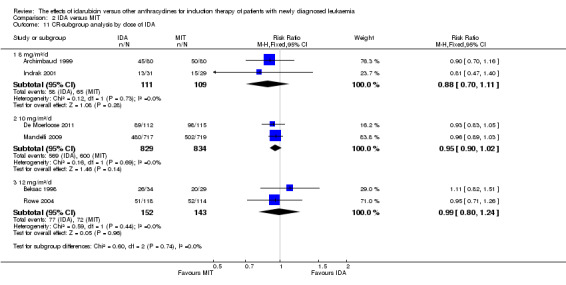
Comparison 2 IDA versus MIT, Outcome 11 CR‐subgroup analysis by dose of IDA.
2.12. Analysis.
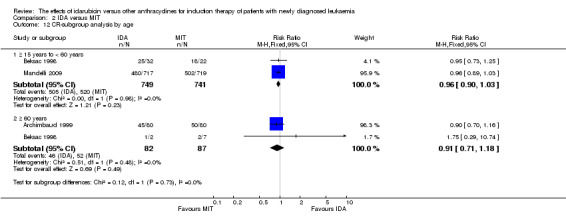
Comparison 2 IDA versus MIT, Outcome 12 CR‐subgroup analysis by age.
2.13. Analysis.
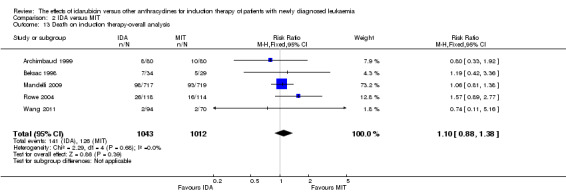
Comparison 2 IDA versus MIT, Outcome 13 Death on induction therapy‐overall analysis.
2.14. Analysis.
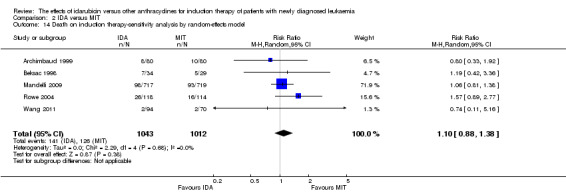
Comparison 2 IDA versus MIT, Outcome 14 Death on induction therapy‐sensitivity analysis by random‐effects model.
2.15. Analysis.
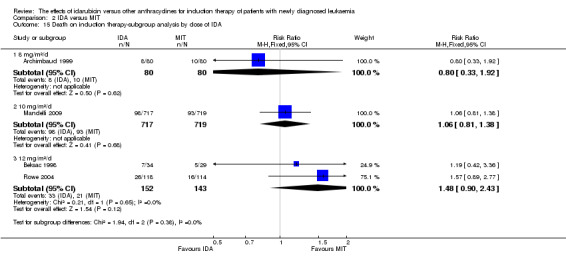
Comparison 2 IDA versus MIT, Outcome 15 Death on induction therapy‐subgroup analysis by dose of IDA.
2.16. Analysis.
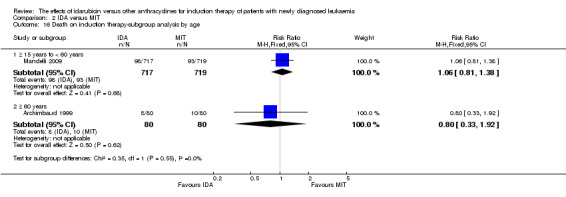
Comparison 2 IDA versus MIT, Outcome 16 Death on induction therapy‐subgroup analysis by age.
2.17. Analysis.
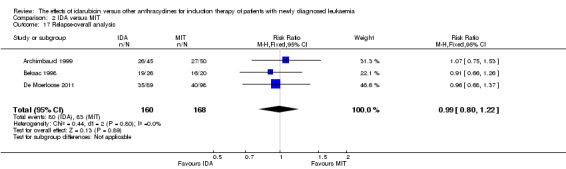
Comparison 2 IDA versus MIT, Outcome 17 Relapse‐overall analysis.
2.18. Analysis.
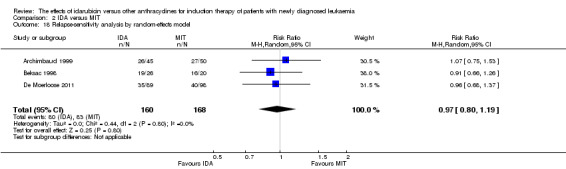
Comparison 2 IDA versus MIT, Outcome 18 Relapse‐sensitivity analysis by random‐effects model.
2.19. Analysis.
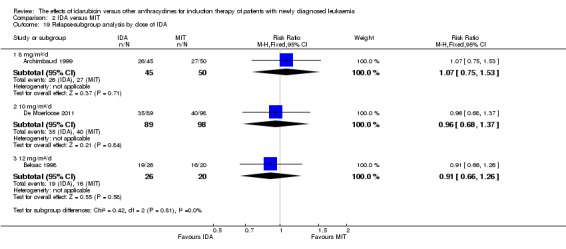
Comparison 2 IDA versus MIT, Outcome 19 Relapse‐subgroup analysis by dose of IDA.
2.20. Analysis.
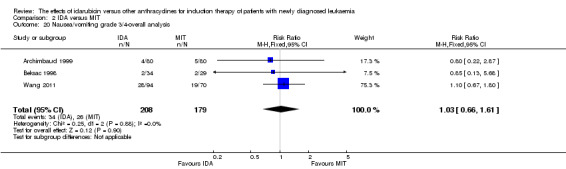
Comparison 2 IDA versus MIT, Outcome 20 Nausea/vomiting grade 3/4‐overall analysis.
2.21. Analysis.
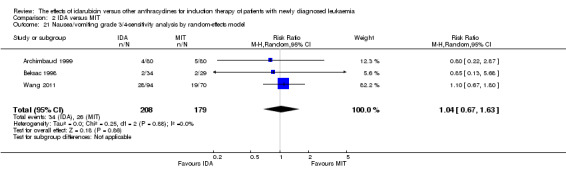
Comparison 2 IDA versus MIT, Outcome 21 Nausea/vomiting grade 3/4‐sensitivity analysis by random‐effects model.
2.22. Analysis.
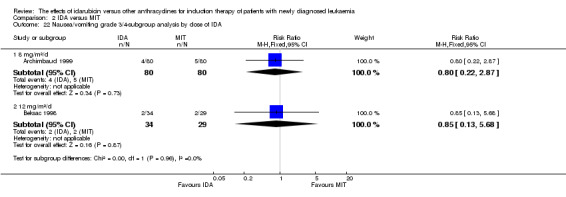
Comparison 2 IDA versus MIT, Outcome 22 Nausea/vomiting grade 3/4‐subgroup analysis by dose of IDA.
2.23. Analysis.
Comparison 2 IDA versus MIT, Outcome 23 Diarrhoea grade 3/4‐overall analysis.
2.24. Analysis.
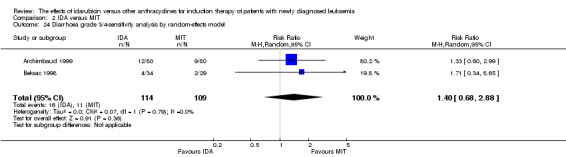
Comparison 2 IDA versus MIT, Outcome 24 Diarrhoea grade 3/4‐sensitivity analysis by random‐effects model.
2.25. Analysis.

Comparison 2 IDA versus MIT, Outcome 25 Diarrhoea grade 3/4‐subgroup analysis by dose of IDA.
2.26. Analysis.
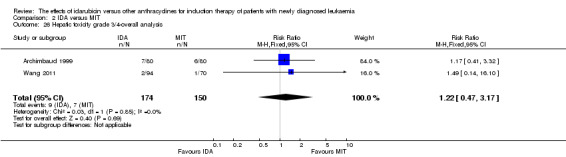
Comparison 2 IDA versus MIT, Outcome 26 Hepatic toxicity grade 3/4‐overall analysis.
2.27. Analysis.
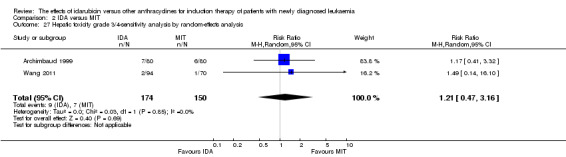
Comparison 2 IDA versus MIT, Outcome 27 Hepatic toxicity grade 3/4‐sensitivity analysis by random‐effects analysis.
2.28. Analysis.
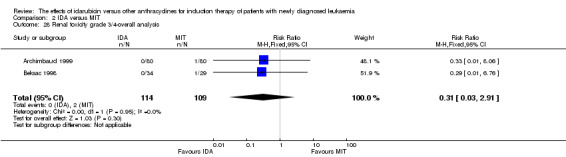
Comparison 2 IDA versus MIT, Outcome 28 Renal toxicity grade 3/4‐overall analysis.
2.29. Analysis.
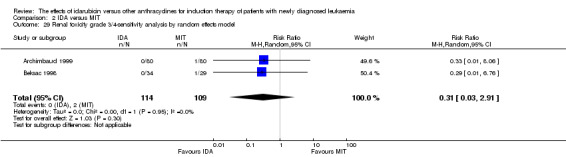
Comparison 2 IDA versus MIT, Outcome 29 Renal toxicity grade 3/4‐sensitivity analysis by random effects model.
2.30. Analysis.
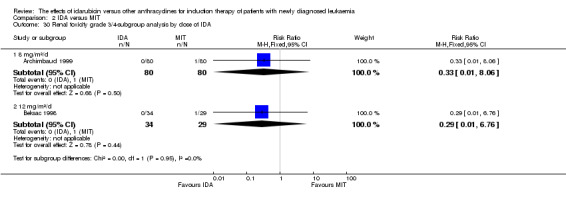
Comparison 2 IDA versus MIT, Outcome 30 Renal toxicity grade 3/4‐subgroup analysis by dose of IDA.
2.31. Analysis.
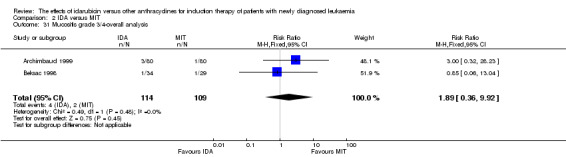
Comparison 2 IDA versus MIT, Outcome 31 Mucositis grade 3/4‐overall analysis.
2.32. Analysis.
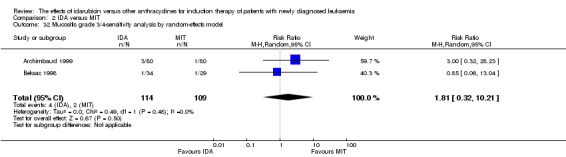
Comparison 2 IDA versus MIT, Outcome 32 Mucositis grade 3/4‐sensitivity analysis by random‐effects model.
2.33. Analysis.
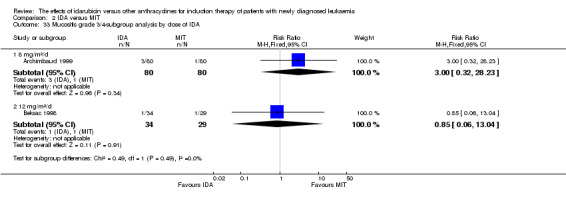
Comparison 2 IDA versus MIT, Outcome 33 Mucositis grade 3/4‐subgroup analysis by dose of IDA.
2.34. Analysis.
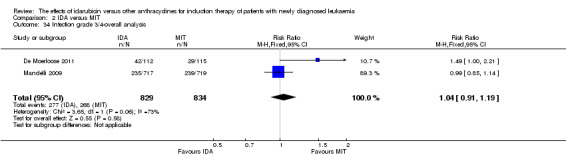
Comparison 2 IDA versus MIT, Outcome 34 Infection grade 3/4‐overall analysis.
2.35. Analysis.
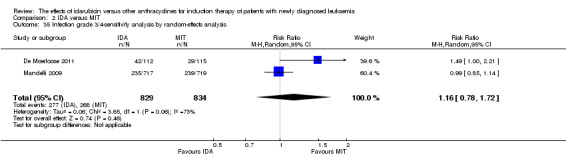
Comparison 2 IDA versus MIT, Outcome 35 Infection grade 3/4‐sensitivity analysis by random‐effects analysis.
Comparison 3. IDA versus DOX.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CR‐overall analysis | 2 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.03, 1.59] |
| 2 CR‐sensitivity analysis by random‐effects analysis | 2 | 187 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.04, 1.60] |
| 3 CR‐subgroup analysis by dose of IDA | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 12 mg/m²/d | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.06, 1.95] |
| 3.2 20 mg/m²/d | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.85, 1.57] |
| 4 CR‐subgroup by AML subtype | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 APL | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.50, 2.00] |
| 4.2 non‐APL AML | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.83, 1.62] |
3.1. Analysis.
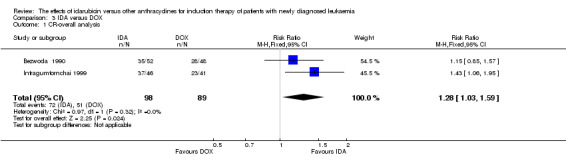
Comparison 3 IDA versus DOX, Outcome 1 CR‐overall analysis.
3.2. Analysis.
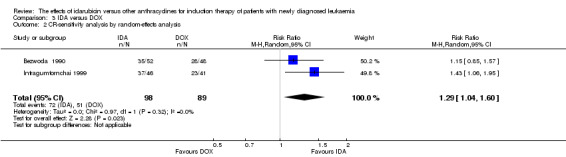
Comparison 3 IDA versus DOX, Outcome 2 CR‐sensitivity analysis by random‐effects analysis.
3.3. Analysis.
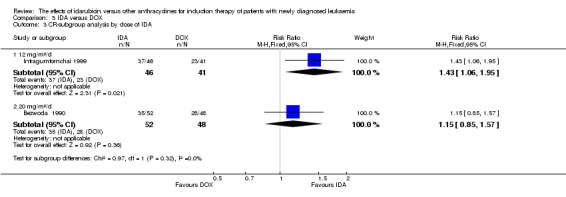
Comparison 3 IDA versus DOX, Outcome 3 CR‐subgroup analysis by dose of IDA.
3.4. Analysis.
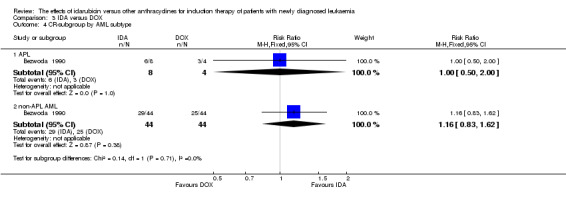
Comparison 3 IDA versus DOX, Outcome 4 CR‐subgroup by AML subtype.
Comparison 4. IDA versus ZRB.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CR‐overall analysis | 2 | 964 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.96, 1.13] |
| 2 CR‐sensitivity analysis by random‐effects model | 2 | 964 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.89, 1.33] |
| 3 Death on induction therapy‐overall analysis | 2 | 964 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.50, 1.13] |
| 4 Death on induction therapy‐sensitivity analysis by random‐effects model | 2 | 964 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.45, 1.20] |
4.1. Analysis.
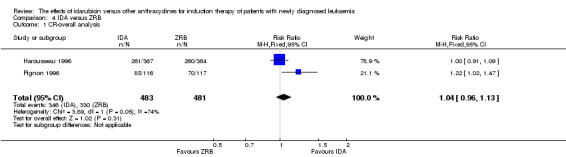
Comparison 4 IDA versus ZRB, Outcome 1 CR‐overall analysis.
4.2. Analysis.
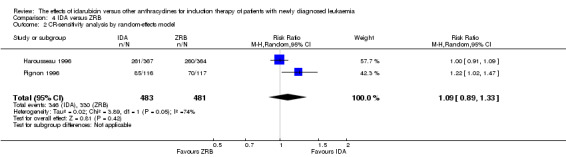
Comparison 4 IDA versus ZRB, Outcome 2 CR‐sensitivity analysis by random‐effects model.
4.3. Analysis.
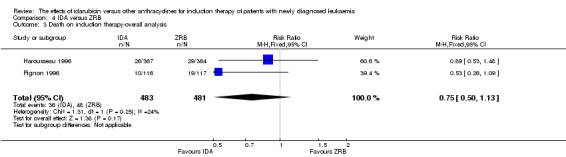
Comparison 4 IDA versus ZRB, Outcome 3 Death on induction therapy‐overall analysis.
4.4. Analysis.
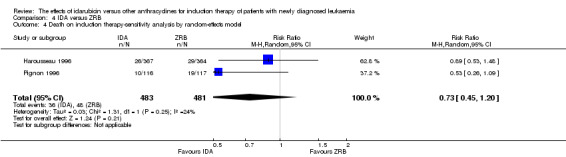
Comparison 4 IDA versus ZRB, Outcome 4 Death on induction therapy‐sensitivity analysis by random‐effects model.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Archimbaud 1999.
| Methods | Design:
Recruitment period:
Median follow‐up:
|
|
| Participants | Eligibility criteria:
Patients randomised (n =160):
Medain age:
Gender (male, female):
Country:
|
|
| Interventions | IDA arm: IAE regimen, 1 to 2 cycles
MIT arm: MAE regimen, 1 to 2 cycles
|
|
| Outcomes | Outcomes and time‐points from the study that are considered in the review:
|
|
| Notes | Published as a journal article Funding: not stated No conflict of interest statement |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomised to" Comment: the study probably had an adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Overall survival | Low risk | Comment: the review authors judge that the outcome OS is unlikely to be influenced by lack of blinding |
| Blinding (performance bias and detection bias) All other outcomes | High risk | Comment: blinding was not explicitly stated |
| Incomplete outcome data (attrition bias) OS and DFS | Low risk | Quote: "All patients randomised were included in the analysis, although four of them (three in the idarubicin and one in the mitoxantrone group) died early, within the 7 days of induction treatment" Comment: all randomised patients were assessed in the analyses |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study has no registered study protocol. The review authors have no information to permit judgement |
| Other bias | Unclear risk | No information provided |
Beksac 1998.
| Methods | Design:
Recruitment period:
Median follow‐up:
|
|
| Participants | Eligibility criteria:
Patients randomised (n = 99):
Medain age:
Gender (Male, female):
Country:
|
|
| Interventions | IDA arm: IA regimen, 1 to 2 cycles
DNR arm (not included in the review): DAE regimen, 1 to 2 cycles
MIT arm: MA regimen, 1 to 2 cycles
|
|
| Outcomes | Outcomes and time‐points from the study that are considered in the review:
|
|
| Notes | Published as a journal article Funded in part by Turksin Academy of Sciences No conflict of interest statement |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly assigned to" Comment: the study probably had an adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Overall survival | Low risk | Comment: the review authors judge that the outcome OS is unlikely to be influenced by lack of blinding |
| Blinding (performance bias and detection bias) All other outcomes | High risk | Comment: blinding was not explicitly stated |
| Incomplete outcome data (attrition bias) OS and DFS | Low risk | Quote: "patients receiving transplants were excluded at the time of transplantation", "Auto/allografts BMT: IDA arm, n = 3; DNR arm, n = 7; MIT arm, n = 4; P value NS" Comment: the small number of missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study has no registered study protocol. The review authors have no information to permit judgement |
| Other bias | Unclear risk | No information provided |
Berman 1991.
| Methods | Design:
Recruitment period:
Median follow‐up:
|
|
| Participants | Eligibility criteria: Inclusion criteria:
Exclusion criteria:
Patients randomised (n = 130):
Medain age:
Gender (male, female):
Country:
|
|
| Interventions | IDA arm: IA regimen, 1 to 2 cycles
DNR arm: DA regimen, 1 to 2 cycles
|
|
| Outcomes | Outcomes and time‐points from the study that are considered in the review:
|
|
| Notes | Published as a journal article Funded in part by American Cancer Society and Adria Laboratories, Dublin, Ohio No conflict of interest statement Updated data were published in 1997 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized all eligible adult patients (...) to" Comment: the study probably had an adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Overall survival | Low risk | Comment: the review authors judge that the outcome OS is unlikely to be influenced by lack of blinding |
| Blinding (performance bias and detection bias) All other outcomes | High risk | Comment: blinding was not explicitly stated |
| Incomplete outcome data (attrition bias) OS and DFS | Low risk | Quote: "A total of 130 patients, 120 of whom were evaluable, were randomized"; "Ten patients were considered inevaluable for the following reasons: four patients had blast counts < 30% and were reclassified after formal review as having either chronic myelomonocytic leukemia (two patients) or myelodysplastic syndrome (two patients); two patients had blastic phase of chronic myelogenous leukemia; one patient had a preexisting history of polycythaemia vera for 6 months before study; one patient was over the age limit of 60; one patient was considered a major protocol violation on day 14 when his therapy was switched to include vincristine and prednisone; and one patient left the hospital on day 3 of therapy and was subsequently lost to follow up" Comment: as the missing data concern a small proportion of the study population (10 out of 130 patients, 7.7%), we judged this study as low risk of bias for incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study has no registered study protocol. The review authors have no information to permit judgement |
| Other bias | Unclear risk | No information provided |
Bezwoda 1990.
| Methods | Design:
Recruitment period:
Median follow‐up:
|
|
| Participants | Eligibility criteria: Inclusion criteria:
Exclusion criteria:
Patients randomised (n = 104):
Mean age:
Gender (male, female):
Country:
|
|
| Interventions | IDA arm: IA regimen, 2 cycles
DOX arm: DA regimen, 2 cycles
|
|
| Outcomes | Outcomes and time‐points from the study that are considered in the review:
|
|
| Notes | Published as a journal article Funding: not stated No conflict of interest statement |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized" Comment: the study probably had an adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Overall survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All other outcomes | High risk | Comment: blinding was not explicitly stated |
| Incomplete outcome data (attrition bias) OS and DFS | Low risk | Quote: "One hundred and four patients were randomized. One patient was ineligible on account of age (71 years, assigned to ADM/Ara‐C) and 3 patients were considered invaluable because of early death before completion of one course of treatment (1 IDA/Ara‐C; 2 ADM/Ara‐C)" Comment: the small number of missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study has no registered study protocol. The review authors have no information to permit judgement |
| Other bias | Unclear risk | No information provided |
Creutzig 2001.
| Methods | Design:
Recruitment period:
Median follow‐up:
|
|
| Participants | Eligibility criteria: Inclusion criteria:
Exclusion criteria:
Patients randomised (n = 358):
Median age:
Gender (male, female):
Country:
|
|
| Interventions | IDA arm: IAE (defined as IDA plus Ara‐C plus etoposide) regimen, 1 cycle
DNR arm: DAE regimen, 1 cycle
|
|
| Outcomes | Outcomes and time‐points from the study that are considered in the review:
|
|
| Notes | Published as a journal article Funded by Deutsche Krebshilfe No conflict of interest statement |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized to" Comment: the study probably had an adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Overall survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All other outcomes | High risk | Comment: blinding was not explicitly stated |
| Incomplete outcome data (attrition bias) OS and DFS | Low risk | Quote: "Analysis of efficacy data was performed according to the intent‐to‐treat principle"; "Two patients allocated to ADE received AIE and four children allocated to AIE received ADE. However, for the intent‐to‐treat analysis these patients remained in their randomized arm" Comment: all randomised patients were included in the analyses |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study has no registered study protocol. The review authors have no information to permit judgement |
| Other bias | Unclear risk | No information provided |
Creutzig 2013.
| Methods | Design:
Recruitment period:
Median follow‐up:
|
|
| Participants | Eligibility criteria: Inclusion criteria:
Exclusion criteria:
Patients randomised (n = 521):
Median age:
Gender (male, female):
Country:
|
|
| Interventions | IDA arm: IAE regimen, 1 cycle
L‐DNR arm: L‐DAE regimen, 1 cycle
|
|
| Outcomes | Outcomes and time‐points from the study that are considered in the review:
|
|
| Notes | Published as a journal article Funded by Deutsche Krebshilfe e.V. and University Hospital Motol, Prague, Czech Republic Dirk Reinhardt is a member of the advisory board from Galen. All other authors declared no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "521 were randomized" Comment: the study probably had an adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Overall survival | Low risk | Comment: the review authors judge that the outcome OS is unlikely to be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) All other outcomes | High risk | Comment: blinding was not explicitly stated |
| Incomplete outcome data (attrition bias) OS and DFS | Low risk | Comment: all randomised patients were included in the analyses |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study has no registered study protocol. The review authors have no information to permit judgement |
| Other bias | Unclear risk | No information provided |
De Moerloose 2011.
| Methods | Design:
Recruitment period:
Median follow‐up:
|
|
| Participants | Eligibility criteria: Inclusion criteria:
Exclusion criteria:
Patients randomised (n = 227):
Median age:
Gender (male, female):
Country:
|
|
| Interventions | IDA arm: 2 cycles First cycle: IAE regimen
Second cycle: IA regimen
MIT arm: 2 cycles First cycle: MAE regimen
Second cycle: MA regimen
|
|
| Outcomes | Outcomes and time‐points from the study that are considered in the review:
|
|
| Notes | Published as an abstract Funded in part by Te´le´vie 2001 and the National Cancer Institute The authors declared no potential conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "pts were randomly assigned to" Comment: the study probably had an adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Overall survival | Low risk | Comment: the review authors judge that the outcome OS is unlikely to be influenced by whether or not blinding. |
| Blinding (performance bias and detection bias) All other outcomes | High risk | Comment: blinding was not explicitly stated |
| Incomplete outcome data (attrition bias) OS and DFS | Unclear risk | Comment: the information about completeness of outcome data is insufficient to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Comment: A study protocol is available (clinical.trials.gov: NCT00002517), but it does not provide any information of the pre‐planned primary and secondary outcomes. |
| Other bias | Unclear risk | No information provided |
Eridani 1989.
| Methods | Design:
Recruitment period:
Median follow‐up:
|
|
| Participants | Eligibility criteria:
Patients randomised (n = 24):
Median age: 58 years (range: 29 to 78 years)
Gender (male, female):
Country:
|
|
| Interventions | Under 60 years: IDA arm: IA regimen, 1‐2 cycles
DNR arm: DA regimen, 1‐2 cycles
Over 60 years: IDA arm: IA regimen, 1‐2 cycles
DNR arm: DA regimen, 1‐2 cycles
|
|
| Outcomes | Outcomes and time‐points from the study that are considered in the review:
|
|
| Notes | Published as a journal article Farmitalia Carlo‐Erba, Milano Italy supplied IDA No conflict of interest statement |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised to" Comment: the study probably had an adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Overall survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All other outcomes | High risk | Comment: blinding was not explicitly stated |
| Incomplete outcome data (attrition bias) OS and DFS | Low risk | Comment: all randomised patients were included in the analyses |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study has no registered study protocol. The review authors have no information to permit judgement |
| Other bias | Unclear risk | No information provided |
Feng 2010.
| Methods | Design:
Recruitment period:
Median follow‐up:
|
|
| Participants | Eligibility criteria:
Patients randomised (n = 105):
Mean age:
Gender (male, female):
Country:
|
|
| Interventions | IDA arm: IA regimen, 2 cycles
DNR arm: DA regimen, 2 cycles
MIT arm: MA regimen, 2 cycles
|
|
| Outcomes | Outcomes and time‐points from the study that are considered in the review:
|
|
| Notes | Published as a journal article in Chinese Funding: not stated No conflict of interest statement |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly assigned to" Comment: the study probably had an adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Overall survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All other outcomes | High risk | Comment: blinding was not explicitly stated |
| Incomplete outcome data (attrition bias) OS and DFS | Low risk | Comment: all randomised patients were included in the analyses |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study has no registered study protocol. The review authors have no information to permit judgement |
| Other bias | Unclear risk | No information provided |
Gardin 2007.
| Methods | Design:
Recruitment period:
Median follow‐up:
|
|
| Participants | Eligibility criteria: Inclusion criteria:
Exclusion criteria:
Patients randomised (n = 416):
Median age:
Gender (male, female):
Country:
|
|
| Interventions | IDA arm: IA regimen, 1 cycle
DNR arm: DA regimen, 1 cycle
|
|
| Outcomes | Outcomes and time‐points from the study that are considered in the review:
|
|
| Notes | Published as a journal article Funded in part by Assistance Publique‐Hopitaux de Paris and Programme Hospitalier de Recherche Clinique The authors declared no potential conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized (...) to" Comment: the study probably had an adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Overall survival | Low risk | Comment: the review authors judge that the outcome OS is unlikely to be influenced by whether or not blinding |
| Blinding (performance bias and detection bias) All other outcomes | High risk | Quote (from registered protocol): "Open Label" Comment: patient and physician unblinded |
| Incomplete outcome data (attrition bias) OS and DFS | Low risk | Comment: all randomised patients were assessed in the analyses |
| Selective reporting (reporting bias) | Low risk | Comment: protocol is available (ClinicalTrials.gov: NCT00363025) Pre‐defined outcomes (relevant for the review) that were reported:
Pre‐defined outcomes (relevant for the review) that were not reported:
|
| Other bias | Unclear risk | No information provided |
Harousseau 1996.
| Methods | Design:
Recruitment period:
Median follow‐up:
|
|
| Participants | Eligibility criteria: Inclusion criteria:
Exclusion criteria:
Patients randomised, analysed (n = 786)
Median age:
Gendar (male, female):
Country:
|
|
| Interventions | IDA arm: IA regimen, 1 to 2 cycles First cycle:
Second cycle:
ZRB arm: ZA regimen, 1 to 2 cycles First cycle:
Second cycle:
|
|
| Outcomes | Outcomes and time‐points from the study that are considered in the review:
|
|
| Notes | Published as a journal article Funded by Programme Hospitalier de Recherche Clinique No conflict of interest statement |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients (...) were randomized between" Comment: the study probably had an adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Overall survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All other outcomes | High risk | Comment: blinding was not explicitly stated |
| Incomplete outcome data (attrition bias) OS and DFS | Low risk | Quote: "A total of 786 patients were included into the study by 16 institutions (IDR: 393, ZRB: 393). However 28 patients were considered ineligible (IDR: 11, ZRB: 17), 16 for an inadequate diagnosis, 8 because they were off age limits and 4 for other ineligibility criteria. Moreover, 27 patients were unable to evaluate for remission induction treatment, 9 because they died before the first day of treatment, 13 because of major protocol violation, 4 because of wrong randomisation, 1 because of missing data" Comment: as the missing data concern a small proportion of the study population (55 out of 786 patients, 7.0%), we judged this study as low risk of bias for incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study has no registered study protocol. The review authors have no information to permit judgement |
| Other bias | Unclear risk | No information provided |
Indrak 2001.
| Methods | Design:
Recruitment period:
Median follow‐up:
|
|
| Participants | Eligibility criteria: Inclusion criteria:
Patients randomised, analysed (n = 60)
Median age:
Gendar (male, female):
Country:
|
|
| Interventions | IDA arm: IA regimen, 1 to 2 cycles
MIT arm: MA regimen, 1 to 2 cycles
|
|
| Outcomes | Outcomes and time‐points from the study that are considered in the review:
|
|
| Notes | Published as a journal article in Czech | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a prospective multicenter randomized study" Comment: the study probably had an adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Overall survival | Low risk | Comment: the review authors judge that the outcome OS is unlikely to be influenced by whether or not blinding |
| Blinding (performance bias and detection bias) All other outcomes | High risk | Comment: blinding was not explicitly stated |
| Incomplete outcome data (attrition bias) OS and DFS | Low risk | Comment: all randomised patients were included in the analyses |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study has no registered study protocol. The review authors have no information to permit judgement |
| Other bias | Unclear risk | No information provided |
Intragumtornchai 1999.
| Methods | Design:
Recruitment period:
Median follow‐up:
|
|
| Participants | Eligibility criteria:
Patients randomised (n = 107)
Median age:
Gender (male, female):
Country:
|
|
| Interventions | IDA arm: IA regimen, 1 cycle
DOX arm: DA regimen, 1 cycle
|
|
| Outcomes | Outcomes and time‐points from the study that are considered in the review:
|
|
| Notes | Published as an abstract Funding: not stated No conflict of interest statement |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients (...) were assigned randomly to" Comment: the study probably had an adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Overall survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All other outcomes | High risk | Comment: blinding was not explicitly stated |
| Incomplete outcome data (attrition bias) OS and DFS | High risk | Quote: "Fifty‐four patients were assigned to receive IDR and 53 to DXR. Three patients in the DXR arm were excluded (2. AML, M3, I, ALL). Bone marrow examination was not available in 17 patients ( 8 in IDR and 9 in DXR arm) which were due to death from uncontrolled infection at time of severe granulocytopenia (6, IDR; 6, DXR), stroke on day 13 (1, DXR), hyperleukocytosis on day 2 (1, IDR)and loss to follow‐up (1, IDR; 2, DXR)" Comment: Outcome data in 20 patients (19%) were not available. The review authors judge that this proportion of missing data will be highly possible to induce bias |
| Selective reporting (reporting bias) | Unclear risk | Comment: The study has no registered study protocol. The review authors have no information to permit judgement |
| Other bias | Unclear risk | No information provided |
Jia 2011.
| Methods | Design:
Recruitment period:
Median follow‐up:
|
|
| Participants | Eligibility criteria:
Patients randomised (n = 68)
Median age:
Gender (male, female):
Country:
|
|
| Interventions | For AML: IDA arm: IA regimen, 2 cycles
DNR arm: DA regimen, 2 cycles
|
|
| Outcomes | Outcomes and time‐points from the study that are considered in the review:
|
|
| Notes | Subtype data for AML were provided only for CRR Published as a journal article in Chinese Funding: no information provided No conflict of interest statement |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random number table method" Comment: the study probably had an adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Overall survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All other outcomes | High risk | Comment: blinding was not explicitly stated |
| Incomplete outcome data (attrition bias) OS and DFS | Low risk | Comment: All randomised patients were assessed in the analyses |
| Selective reporting (reporting bias) | Unclear risk | Comment: The study has no registered study protocol. The review authors have no information to permit judgement |
| Other bias | Unclear risk | No information provided |
Lee 2012.
| Methods | Design:
Recruitment period:
Median follow‐up:
|
|
| Participants | Eligibility criteria:
Patients randomised (n = 157)
Median age:
Gender (male, female):
Country:
|
|
| Interventions | IDA arm: IA regimen, 1 cycle
DNR arm: DA regimen, 1 cycle
|
|
| Outcomes | Outcomes and time‐points from the study that are considered in the review:
|
|
| Notes | An interim analysis Published as an abstract Funding: no information provided No conflict of interest statement |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random assignments" Comment: the study probably had an adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Overall survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All other outcomes | High risk | Quote (from registered protocol): "Open Label" Comment: patient and physician unblinded |
| Incomplete outcome data (attrition bias) OS and DFS | Low risk | Comment: all randomised patients were assessed in the analyses |
| Selective reporting (reporting bias) | High risk | Comment: the study is published as an abstract Comment: protocol is available (ClinicalTrials.gov: NCT01145846) Pre‐defined outcomes (relevant for the review) that were reported:
Pre‐defined outcomes (relevant for the review) that were not reported:
|
| Other bias | Unclear risk | No information provided |
Mandelli 1991.
| Methods | Design:
Recruitment period:
Median follow‐up:
|
|
| Participants | Eligibility criteria: Inclusion criteria:
Exclusion criteria:
Patients randomised (n = 249)
Median age:
Genger (male, female):
Country:
|
|
| Interventions | IDA arm: IA regimen, 1 to 2 cycles First cycle:
Second cycle:
DNR arm: DA regimen, 1 to 2 cycles First cycle:
Second cycle:
|
|
| Outcomes | Outcomes and time‐points from the study that are considered in the review:
|
|
| Notes | Published as a journal article Funding: not stated IDA was supplied by Farmitalia‐Carlo Erba Research Laboratories, Milan, Italy No conflict of interest statement |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomised to" Comment: the study probably had an adequate sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "centrally randomised" |
| Blinding (performance bias and detection bias) Overall survival | Low risk | Comment: the review authors judge that the outcome OS is unlikely to be influenced by lack of blinding |
| Blinding (performance bias and detection bias) All other outcomes | High risk | Comment: blinding was not explicitly stated |
| Incomplete outcome data (attrition bias) OS and DFS | Low risk | Quote: "all randomised patients were evaluated for the efficacy of the treatment on the basis of an "intention to treat"" Comment: all randomised patients were included in the analyses |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study has no registered study protocol. The review authors have no information to permit judgement |
| Other bias | Unclear risk | No information provided |
Mandelli 2009.
| Methods | Design:
Recruitment period:
Median follow‐up:
|
|
| Participants | Eligibility criteria: Inclusion criteria:
Exclusion criteria:
Patients randomised (n = 2157)
Median age:
Gender (male, female):
Country:
|
|
| Interventions | IDA arm: IAE regimen, 1 to 2 cycles
DNR arm: DAE regimen, 1 to 2 cycles
MIT arm: MIE regimen, 1 to 2 cycles
|
|
| Outcomes | Outcomes and time‐points from the study that are considered in the review:
|
|
| Notes | Published as a journal article Funded by National Cancer Institute, Bethesda, MD, Italian Cancer League and Italian Association Against Leukemias, Lymphoma, and Myeloma The authors declared no potential conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly assigned" Comment: the study probably had an adequate sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were screened at each centre, and those who fulfilled the eligibility criteria were randomly assigned at the European Organisation for Research and Treatment of Cancer Data Center in Brussels, Belgium" |
| Blinding (performance bias and detection bias) Overall survival | Low risk | Comment: the review authors judge that the outcome OS is unlikely to be influenced by lack of blinding |
| Blinding (performance bias and detection bias) All other outcomes | High risk | Comment: blinding was not explicitly stated |
| Incomplete outcome data (attrition bias) OS and DFS | Low risk | Quote: "All the efficacy analyses were performed according to the intention‐to‐treat‐principle (all patients randomly assigned were included)" Comment: all randomised patients were included in the analyses |
| Selective reporting (reporting bias) | Unclear risk | Comment: a study protocol is available (clinical.trials.gov: NCT00002549), but it does not provide any information of the pre‐planned primary and secondary outcomes. |
| Other bias | Unclear risk | No information provided |
Masaoka 1996.
| Methods | Design:
Recruitment period:
Median follow‐up:
|
|
| Participants | Eligibility criteria:
Patients randomised (n = 72)
Median age:
Gender (male, female):
Country:
|
|
| Interventions | IDA arm: IA regimen, 1 to 2 cycles
DNR arm: DA regimen, 1 to 2 cycles
|
|
| Outcomes | Outcomes and time‐points from the study that are considered in the review:
|
|
| Notes | Published as a journal article Funding: not stated No conflict of interest statement |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were allocated by dynamic randomisation" Comment: the study probably had an adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Overall survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All other outcomes | High risk | Comment: blinding was not explicitly stated |
| Incomplete outcome data (attrition bias) OS and DFS | Low risk | Quote: "the reasons for he study withdrawal from the two treatment arms are as follows: no drug administration, one IDA arm, one DNR arm; previous DNR treatment, two IDA; chronic myeloid leukaemia blastic crisis, one IDA; ALL, one DNR; early death, one DNR; and protocol violation, one DNR" Comment: the small number of missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study has no registered study protocol. The review authors have no information to permit judgement |
| Other bias | High risk | Comment: baseline characteristics of patients were imbalanced in gender. Male/female in IDA and DNR arms were 23/9 and 13/19 respectively (P value = 0.023) |
Ohtake 2011.
| Methods | Design:
Recruitment period:
Median follow‐up:
|
|
| Participants | Eligibility criteria: Inclusion criteria:
.Exclusion criteria:
Patients randomised (n = 1057)
Median age:
Gender (male, female):
Country:
|
|
| Interventions | IDA arm: IA regimen, 1 to 2 cycles
DNR arm: DA regimen, 1 to 2 cycles
|
|
| Outcomes | Outcomes and time‐points from the study that are considered in the review:
|
|
| Notes | Published as a journal article Funded in part by Ministry of Health, Labour, and Welfare of Japan The authors declared no potential conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly assigned by use of a centralized computer system to" Comment: the study probably had an adequate sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "patients were randomly assigned by use of a centralized computer system to" |
| Blinding (performance bias and detection bias) Overall survival | Low risk | Comment: the review authors judge that the outcome OS is unlikely to be influenced by lack of blinding |
| Blinding (performance bias and detection bias) All other outcomes | High risk | Quote (from registered protocol): "Open‐no one is blinded" Comment: patient and physician unblinded |
| Incomplete outcome data (attrition bias) OS and DFS | Low risk | Quote: "all analyses were performed according to the intention‐to‐treat principle" Comment: all randomised patients were included in the analyses |
| Selective reporting (reporting bias) | Low risk | Comment: protocol is available (UMIN‐CTR Clinical Trial: C000000157) Pre‐defined outcomes (relevant for the review) that were reported:
Pre‐defined outcomes (relevant for the review) that were not reported:
|
| Other bias | Unclear risk | No information provided |
Pautas 2010.
| Methods | Design:
Recruitment period:
Median follow‐up:
|
|
| Participants | Eligibility criteria: Inclusion criteria:
Exclusion criteria:
Patients randomised (n = 478)
Median age:
Gender (male, female):
Country:
|
|
| Interventions | IDA3 arm: IA regimen, 1 cycle
IDA4 arm: IA regimen, 1 cycle
DNR arm: DA regimen, 1 cycle
|
|
| Outcomes | Outcomes and time‐points from the study that are considered in the review:
|
|
| Notes | Published as a journal article Funded in part by Assistance Publique‐Hopitaux de Paris and the Programme Hospitalier de Recherche Clinique The authors declared no potential conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly assigned to" Comment: the study probably had an adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Overall survival | Low risk | Comment: the review authors judge that the outcome OS is unlikely to be influenced by lack of blinding |
| Blinding (performance bias and detection bias) All other outcomes | High risk | Quote (from registered protocol): "Open Label" Comment: patient and physician unblinded |
| Incomplete outcome data (attrition bias) OS and DFS | Low risk | Quote: "statistical analysis was performed on an intention‐to‐treat basis, using three‐arm comparisons"; "478 patients were included (Fig 1). Ten patients were excluded for misdiagnosis. Final analysis included 468 patients"; "Excluded: misdiagnosed DNR (n = 4) IDA3 (n = 5) IDA4 (n = 1)" Comment: the small number of missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Comment: protocol is available (ClinicalTrial.gov: NCT00931138) Pre‐defined outcomes (relevant for the review) that were reported:
Pre‐defined outcomes (relevant for the review) that were not reported:
Reported outcomes (relevant for the review) that were not predefined in the protocol:
|
| Other bias | Unclear risk | No information provided |
Pignon 1996.
| Methods | Design:
Recruitment period:
Median follow‐up:
|
|
| Participants | Eligibility criteria:
Patients randomised (n = 251)
Median age:
Gender (male, female):
Country:
|
|
| Interventions | IDA arm: IA regimen, 1 to 2 cycles First cycle:
Second cycle:
ZRB arm: ZA regimen, 1 to 2 cycles First cycle:
Second cycle:
|
|
| Outcomes | Outcomes and time‐points from the study that are considered in the review:
|
|
| Notes | Published as a journal article Funding: not stated No conflict of interest statement |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized" Comment: the study probably had an adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Overall survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All other outcomes | High risk | Comment: blinding was not explicitly stated |
| Incomplete outcome data (attrition bias) OS and DFS | Low risk | Quote: "Two hundred and fifty‐one patients were randomized (IDR: 124; ZRB: 127). 10 were ineligible (IDR: five; ZRB: five) for the following reasons: myelodysplastic syndrome: five; acute lymphoblastic leukaemia: two; previous chemotherapy for breast cancer: one; age >65 years: one; abnormal hepatic function: one. Eight patients were unable to evaluate for induction treatment (IDR: two; ZRB: six) because of protocol violation (errors in dosages or type of drugs)." Comment: the small number of missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study has no registered study protocol. The review authors have no information to permit judgement |
| Other bias | High risk | Comment: baseline characteristics of patients were imbalanced in gender. Male/female in IDA and ZRB arms were 70/46 and 52/65 respectively (P value = 0.02) |
Reiffers 1996.
| Methods | Design:
Recruitment period:
Median follow‐up:
|
|
| Participants | Eligibility criteria: Inclusion criteria:
Exclusion criteria:
Patients randomised (n = 220)
Median age:
Gender (male, female):
Country:
|
|
| Interventions | IDA arm: IA regimen, 1 to 2 cycles
DNR arm: DA regimen, 1 to 2 cycles
|
|
| Outcomes | Outcomes and time‐points from the study that are considered in the review:
|
|
| Notes | Published as a journal article Funding: not stated No conflict of interest statement |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized to" Comment: the study probably had an adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Overall survival | Low risk | Comment: the review authors judge that the outcome OS is unlikely to be influenced by lack of blinding |
| Blinding (performance bias and detection bias) All other outcomes | High risk | Comment: blinding was not explicitly stated |
| Incomplete outcome data (attrition bias) OS and DFS | Low risk | Comment: all randomised patients were included in the analyses |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study has no registered study protocol. The review authors have no information to permit judgement |
| Other bias | Unclear risk | No information provided |
Rowe 2004.
| Methods | Design:
Recruitment period:
Median follow‐up:
|
|
| Participants | Eligibility criteria:
Patients randomised (n = 362)
Median age:
Gender (male, female):
Country:
|
|
| Interventions | IDA arm: IA regimen, 1 to 2 cycles
DNR arm: DA regimen, 1 to 2 cycles
MIT arm: MA regimen, 1 to 2 cycles
|
|
| Outcomes | Outcomes and time‐points from the study that are considered in the review:
|
|
| Notes | Published as a journal article Funded in part by National Cancer Institute, National Institutes of Health, and the Department of Health and Human Services No conflict of interest statement |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized to" Comment: the study probably had an adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Overall survival | Low risk | Comment: the review authors judge that the outcome OS is unlikely to be influenced by lack of blinding |
| Blinding (performance bias and detection bias) All other outcomes | High risk | Comment: blinding was not explicitly stated |
| Incomplete outcome data (attrition bias) OS and DFS | Low risk | Quote: "There were 122 patients randomized to DA, 121 to IA, and 119 to MA"; "Of the 362 randomized patients, 348 were medically eligible with a centrally reviewed diagnosis and submitted data; these patients were included in the primary efficacy comparison of these treatment arms (116 on DA, 118 on IA, and 114 on MA)" Comment: the small number of missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study has no registered study protocol. The review authors have no information to permit judgement |
| Other bias | Unclear risk | No information provided |
Récher 2014.
| Methods | Design:
Recruitment period:
Median follow‐up:
|
|
| Participants | Eligibility criteria: Inclusion criteria:
Exclusion criteria:
Patients randomised (n = 832):
Median age:
Gender (male, female):
Country: France, 28 centres |
|
| Interventions | IDA arm: IA (defined as IDA plus Ara‐C) regimen, 1‐2 cycles First cycle:
Second cycle:
DNR arm: DA regimen, 1‐2 cycles First cycle:
Second cycle:
|
|
| Outcomes | Outcomes and time‐points from the study that are considered in the review:
|
|
| Notes | Published as a journal article Funding by the Centre Hospitalier de Nantes, France The authors declared no potential conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients were randomly assigned to patients" Comment: the study probably had an adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Overall survival | Low risk | Comment: the review authors judge that the outcome OS is unlikely to be influenced by lack of blinding |
| Blinding (performance bias and detection bias) All other outcomes | High risk | Quote (from registered protocol): "Open Label". Comment: patient and physician unblinded |
| Incomplete outcome data (attrition bias) OS and DFS | Low risk | Quote:"Of the 832 patients enrolled in the
study, 14 were excluded"; " 8 patients with wrong diagnosis and 1 death before induction" in IDA arm and "5 received idarubicin" in DNR arm. Comment: as the missing data concern a small proportion of the study population (14 out of 832 patients, 1.7%), we judged this study as low risk of bias for incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: A study protocol is available (clinical.trials.gov: NCT01015196), but it does not provide any information of the pre‐planned primary and secondary outcomes. |
| Other bias | Unclear risk | No information provided |
Vogler 1992.
| Methods | Design:
Recruitment period:
Median follow‐up:
|
|
| Participants | Eligibility criteria:
Patients randomised (n = 230)
Median age:
Gender (male, female):
Country:
|
|
| Interventions | IDA arm: IA regimen, 1 to 2 cycles
DNR arm: DA regimen, 1 to 2 cycles
|
|
| Outcomes | Outcomes and time‐points from the study that are considered in the review:
|
|
| Notes | Published as a journal article Funded in part by Adria Laboratories, the manufacturer of idarubicin No conflict of interest statement Updated data were published in 1997 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were assigned randomly to" Comment: the study probably had an adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Overall survival | Low risk | Comment: the review authors judge that the outcome OS is unlikely to be influenced by lack of blinding |
| Blinding (performance bias and detection bias) All other outcomes | High risk | Comment: blinding was not explicitly stated |
| Incomplete outcome data (attrition bias) OS and DFS | Low risk | Quote: "No. of patients randomized: IDR 111, DNR 119", "Exclusions: Wrong diagnosis: IDR 2, DNR 1; Protocol violations: IDR 4, DNR 2; Randomized, not treated: DNR 1; Died before treatment: DNR 2" Comment: the small number of missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study has no registered study protocol. The review authors have no information to permit judgement |
| Other bias | High risk | Comment: baseline characteristics of patients were imbalanced in platelet count. The median platelet count was significantly higher on the IDA arm (P value = 0.023) |
Wang 2011.
| Methods | Design:
Recruitment period:
Median follow‐up:
|
|
| Participants | Eligibility criteria:
Patients randomised (n = 164)
Median age:
Gender (male, female):
Country:
|
|
| Interventions | IDA arm: IA regimen, 1 cycle
MIT arm: MA regimen, 1 cycle
|
|
| Outcomes | Outcomes and time‐points from the study that are considered in the review:
|
|
| Notes | Published as a journal article in Chiese Funding: not stated No conflict of interest statement |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized to" Comment: the study probably had an adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Overall survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All other outcomes | High risk | Comment: blinding was not explicitly stated |
| Incomplete outcome data (attrition bias) OS and DFS | Low risk | Comment: all randomised patients were assessed in the analyses |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study has no registered study protocol. The review authors have no information to permit judgement |
| Other bias | Unclear risk | No information provided |
Wiernik 1992.
| Methods | Design:
Recruitment period:
Median follow‐up:
|
|
| Participants | Eligibility criteria: Inclusion criteria:
Exclusion criteria:
Patients randomised (n = 214)
Median age:
Gender (male, female):
Country:
|
|
| Interventions | IDA arm: IA regimen, 1 to 2 cycles
DNR arm: DA regimen, 1 to 2 cycles
|
|
| Outcomes | Outcomes and time‐points from the study that are considered in the review:
|
|
| Notes | Published as a journal article Funded in part by Adria Laboratories, the manufacturer of idarubicin No conflict of interest statement Updated data were published in 1997 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a total of 214 adults (...) were randomized to" Comment: the study probably had an adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Overall survival | Low risk | Comment: the review authors judge that the outcome OS is unlikely to be influenced by lack of blinding |
| Blinding (performance bias and detection bias) All other outcomes | High risk | Comment: blinding was not explicitly stated |
| Incomplete outcome data (attrition bias) OS and DFS | Low risk | Comment: all randomised patients were included in the analyses |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study has no registered study protocol. The review authors have no information to permit judgement |
| Other bias | Unclear risk | No information provided |
ADE: daunorubicin plus cytarabine plus etoposide; ADM: doxorubicin; AEs: adverse events; AIE: idarubicin plus cytarabine plus etoposide; ALL: acute lymphocytic leukaemia; AML: acute myeloid leukaemia; Ara‐C: cytarabine; BMT: bone marrow transplant; CML: chronic myeloid leukaemia; CNS: central nervous system; CR: complete remission; CRR: complete remission rate; DA: daunorubicin or doxorubicin plus cytarabine; DAE: daunorubicin plus cytarabine plus etoposide; DFS: disease‐free survival; DNR: daunorubicin; DOX: doxorubicin; DXR: doxorubicin; ECOG: Eastern Cooperative Oncology Group; FAB: French‐American‐British; IA: idarubicin plus cytarabine; IAE: idarubicin plus cytarabine plus etoposide; IDA: idarubicin; IV: intravenously; L‐DAE: liposomal daunorubicin plus cytarabine plus etoposide; L‐DNR: liposomal daunorubicin; LVEF: left ventricular ejection fraction; MA: mitoxantrone plus cytarabine; MAE: mitoxantrone plus cytarabine plus etoposide; MDS: myelodysplastic syndrome; MIT: mitoxantrone; NS: not significant; OS: overall survival; PS: performance status; RCT: randomised controlled trial; RR: relapse rate; VP‐16: etoposide; WHO: World Health Organization; ZA: zorubicin plus cytarabine; ZRB: zorubicin.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Belhabri 1999 | Subtype data for newly diagnosed AML patients were not available and most patients were not with newly diagnosed AML (only 5 out of 53 had newly diagnosed AML) |
| Buchner 2012 | Not a RCT |
| Candoni 2009 | Not a RCT |
| Castaigne 2004 | RCT:comparison arms not treated with idarubicin |
| Chan‐Lam 1992 | Not a RCT (comment) |
| Creutzig 2000 | Not a RCT |
| Creutzig 2005 | Not a RCT |
| Dluzniewska 2005 | Not a RCT |
| Gardin 2013 | Not a RCT |
| Keldsen 1990 | Not a RCT |
| Lambertenghi‐Deliliers 1989 | Not a RCT |
| Lange 2008 | Induction was not randomised |
| Leone 1999 | Not a RCT (review) |
| Li 2013a | Not a RCT |
| Liu 2005 | RCT: both arms treated with idarubicin |
| Morita 2010 | Subtype data for newly diagnosed AML patients were not available and most patients were not with newly diagnosed AML (only 54 out of 120 had newly diagnosed AML) |
| O'Brien 2002 | Not a RCT |
| Oriol 2003 | Not a RCT |
| Pashko 1991 | Cost‐effectiveness study of a RCT comparing IDA versus DNR |
| Reinhardt 2005 | Not a RCT (correspondence) |
| Shi 2013 | Not a RCT |
| Volkova 1993 | Not a RCT |
| Wheatley 2001 | Not a RCT (comment) |
| Witz 1995 | Induction was not randomised |
| Xia 2013 | Not a RCT |
AML: acute myeloid leukaemia; DNR: daunorubicin; IDA: idarubicin; RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
C‐022.
| Trial name or title | Idarubicin versus high dose Daunorubicin in AML (NCT01145846) |
| Methods | A Phase III, open Label, randomised trial of idarubicin versus high‐dose daunorubicin in combination with cytarabine in the induction chemotherapy for untreated AML Randomisation:
|
| Participants | Inclusion criteria:
|
| Interventions | IDA arm:
DNR arm:
|
| Outcomes | Outcomes and time‐points from the registered protocol of the study that are considered in the review:
|
| Starting date | May 2010 |
| Contact information | Je‐Hwan Lee, professor, Asan Medical Center (jhlee3@amc.seoul.kr) |
| Notes | Staty status according to ClinicalTrials.gov: this study is currently recruiting participants |
AEs: adverse event; ALT: alanine aminotransferase; AML: acute myeloid leukaemia; Ara‐C: cytarabine; AST: aspartate aminotransferase; CRR: complete remission rate; DFS: disease‐free survival; DNR: daunorubicin; IDA: idarubicin; IV: intravenous; OS: overall survival; PS: performance status; RR: relapse rate.
Differences between protocol and review
The protocol stated that we would perform subgroup analyses by anthracycline agent types of control intervention. To avoid clinical heterogeneity, we did not perform this subgroup analysis but carried out separate meta‐analyses for IDA versus different anthracyclines.
We added a subgroup analysis by total dose of DNR (< 180 mg/m² or ≥ 180 mg/m²).
In the protocol, we stated that we would perform a subgroup analysis by age (< 60 years or ≥60 years). However, we performed the subgroup analysis by age (< 15 years or ≥ 15 years to < 60 years or ≥60 years), which we considered to be more appropriately defined than that in the protocol.
We planned to analyse the outcome of quality of life (QoL). However, this could not be accomplished because none of the included studies reported QoL.
We added the analysis of differences between subgroups using the Chi² test at a significance level of P value < 0.05, which was not mentioned in the protocol.
Contributions of authors
Xi Li: developing protocol, identification of studies, screening studies, extracting data, conducting analysis, drafting report.
ShuangNian Xu: developing protocol, screening studies, extracting data, conducting analysis, revising the protocol and the review.
Ya Tan: identification of studies, screening studies, conducting analysis.
Jieping Chen: developing protocol, identification of studies, screening studies, drafting report, revising the protocol and the review.
Sources of support
Internal sources
No sources of support supplied
External sources
National Natural Science Fund (NSFC, 30971066), China.
National Natural Science Fund (NSFC, 81270605), China.
Third Military Medical University Clinnic and Science Great Fund Project (2012XLC03), China.
Declarations of interest
None known.
New
References
References to studies included in this review
Archimbaud 1999 {published data only}
- Archimbaud E, Jehn U, Thomas X, Cataldo F, Fillet G, Belhabri A, et al. A randomized comparison of idarubicin vs mitoxantrone, in association to VP‐16 and cytarabine for induction/consolidation therapy followed by autologous stem cell transplantation in elderly patients with acute myeloid leukemia (AML). British Journal of Haematology 1996;Suppl 2:133. [Google Scholar]
- Archimbaud E, Jehn U, Thomas X, Cataldo F, Fillet G, Belhabri A, et al. Idarubicin versus mitoxantrone, in association with VP‐16 and ARAC for induction/consolidation therapy followed by autologous stem cell transplantation in elderly patients with acute myeloid leukemia. Final analysis of a randomized trial [abstract]. Journal of Clinical Onclogy 1997:3a.
- Archimbaud E, Jehn U, Thomas X, Cataldo F, Fillet G, Belhabri A, et al. Multicenter randomized phase II trial of idarubicin vs mitoxantrone, combined with VP‐16 and cytarabine for induction/consolidation therapy, followed by a feasibility study of autologous peripheral blood stem cell transplantation in elderly patients with acute myeloid leukemia. Leukemia 1999;13:843‐9. [DOI] [PubMed] [Google Scholar]
- Cataldo F, Martin C, Thomas X. Idarubicin or mitoxantrone, VP‐16 and cytarabine for induction/consolidation therapy followed by autologous stem cell transplantation in elderly patients with acute myeloid leukemia (AML): a feasibility study. Annals of Hematology 1995:a136.
Beksac 1998 {published data only}
- Beksac M, Arslan O, Koc H, Akan H, Ilhan O, Arat M, et al. Randomised unicenter trial for comparison of three regimens in de novo adult acute nonlymphoblastic leukaemia. Medical Oncology 1998;15:183‐90. [DOI] [PubMed] [Google Scholar]
- Beksac M, Arslan O, Koc H, Akan H, Ilhan O, Arat M, et al. A randomized single institutional study on comparison of idarubicin (I) versus daunorubicin (D) versus mitoxantrone (M) containing induction and consolidation regimens in acute myeloblastic leukemia (AML). Blood 1997, issue 10 Suppl 1 (Pt 2):237b.
Berman 1991 {published data only}
- Berman E, Gee TS, Kempin S, Gulati S, Andreeff M, Gabrilove J. Results of a comparative trial of idarubicin and cytarabine with daunorubicin and cytarabine in patients with untreated acute nonlymphocytic leukemia. Blood 1986, issue 5 Suppl 1:218a.
- Berman E, Heller G, Santorsa J, McKenzie S, Gee T, Kempin S, et al. Results of a randomized trial comparing idarubicin and cytosine arabinoside with daunorubicin and cytosine arabinoside in adult patients with newly diagnosed acute myelogenous leukemia. Blood 1991;77:1666‐74. [PubMed] [Google Scholar]
- Berman E, Raymond G, Gee TS, Kempin S, Andreeff M, Gulati S. Results of a randomized trial comparing idarubicin (IDR)/cytosine arabinoside (Ara‐C) with daunorubicin (DNR)/Ara‐C in adult patients (pts) with untreated acute nonlymphocytic leukemia (ANLL). Blood 1988, issue 5 Suppl 1:189a.
- Berman E, Raymond V, Gee T, Kempin SJ, Gulati S, Andreeff M, et al. Idarubicin in acute leukemia: results of studies at Memorial Sloan‐Kettering Cancer Center. Seminars in Oncology 1989;16(1 Suppl 2):30‐4. [PubMed] [Google Scholar]
Bezwoda 1990 {published data only}
- Bezwoda WR, Dansey RD. Idarubicin plus cytarabine versus doxorubicin plus cytarabine in induction therapy for acute non‐lymphoid leukaemia: A randomized trial. Leukemia and Lymphoma 1990;1:221‐5. [DOI] [PubMed] [Google Scholar]
Creutzig 2001 {published data only}
- Creutzig U, Berthold F, Boos J, Fleischhack G, Gadner H, Gnekow A. Improved treatment results in children with AML: Results of study AML‐BFM 93. Klinische Padiatrie 2001;213(4):175‐85. [DOI] [PubMed] [Google Scholar]
- Creutzig U, Hermann J, Gadner H, Zimmermann M, Jurgens H, Ritter J. Comparison of equipotential doses of daunorubicin and idarubicin during induction: First results of the randomized trial BFM 93 in children with AML. Blood 1997, issue 10 Suppl 1 (Pt 1):584a.
- Creutzig U, Ritter J, Zimmermann M, Hermann J, Gadner H, Blutters Sawatzki D, et al. Idarubicin improves blast cell clearance during induction therapy in children with AML: results of study AML‐BFM 93. Leukemia 2001;15:348‐54. [DOI] [PubMed] [Google Scholar]
- Ritter J, Zimmermann M, Creutzig U. Randomization of induction treatment with daunorubicin vs idarubicin in children with AML ‐ Results of study AML‐BFM 93. Annals of Hematology 1999:S1.
Creutzig 2013 {published data only}
- Creutzig U, Zimmermann M, Bourquin J‐P, Dworzak MN, Fleischhack G, Graf N, et al. Randomized trial comparing liposomal daunorubicin with idarubicin as induction for pediatric acute myeloid leukemia: results from Study AML‐BFM 2004. Blood 2013;122:37‐43. [DOI] [PubMed] [Google Scholar]
- Creutzig U, Zimmermann M, Dworzak M, Bourquin JP, Neuhoff C, Sander A. Study AML‐BFM 2004: Improved survival in childhood acute myeloid leukemia without increased toxicity. Blood 2010;116(21):Abstract 181. [Google Scholar]
De Moerloose 2011 {published data only}
- Moerloose B, Suciu S, Munzer M, Piette C, Yakouben K, Margueritte G, et al. Similar efficacy and toxicity profile for idarubicin and mitoxantrone in induction and intensification treatment of children with acute myeloid leukemia (AML) or myelodysplasia (MDS): Long‐term results of the EORTC‐CLG randomized phase III trial 58921. Blood 2011;118:Abstract 2615. [Google Scholar]
- Entz‐Werle N, Suciu S, Werff ten Bosch J, Vilmer E, Bertrand Y, Benoit Y, et al. Results of 58872 and 58921 trials in acute myeloblastic leukemia and relative value of chemotherapy vs allogeneic bone marrow transplantation in first complete remission: the EORTC Children Leukemia Group report. Leukemia 2005;19(12):2072‐81. [DOI] [PubMed] [Google Scholar]
Eridani 1989 {published data only}
- Eridani S, Singh AK, Slater NGP, Pearson TC, Phaure TAJ, Semple MJ, et al. A phase II study of idarubicin versus daunorubicin in the treatment of acute non‐lymphocytic leukaemia. Cancer Journal 1989;2(9):296‐8. [Google Scholar]
Feng 2010 {published data only}
- Feng R, Zhang HX, Li HM. An analysis on clinical response of IA, MA and DA regimens in the treatment for acute myeloid leukemia. Journal of Oncology 2010;16(12):972‐5. [Google Scholar]
Gardin 2007 {published data only}
- Gardin C, Turlure P, Fagot T, Thomas X, Terre C, Contentin N, et al. Postremission treatment of elderly patients with acute myeloid leukemia in first complete remission after intensive induction chemotherapy: Results of the multicenter randomized Acute Leukemia French Association (ALFA) 9803 trial. Blood 2007;109:5129‐35. [DOI] [PubMed] [Google Scholar]
Harousseau 1996 {published data only}
- Harousseau JL. A phase III study comparing idarubicin and rubidazone in combination with cytosine arabinoside in acute myeloid leukemia [abstract]. Haematologica 1991, issue Suppl 4:9.
- Harousseau JL, Pignon B, Witz F, Polin V, Tellier Z, Hurteloup P, et al. Treatment of acute myeloblastic leukemia in adults. The GOELAM experience. Hematology and Cell Therapy 1996;38:381‐91. [DOI] [PubMed] [Google Scholar]
Indrak 2001 {published data only}
- Indrak K, Hubacek J, Mayer J, Voglova J, Jarosova M, Krahulova M, et al. Comparison of the effectiveness of idarubicin (Zavedos) and mitoxantrone (Refador) in induction therapy of acute myeloid leukemia in elderly patients (55‐75) (a prospective multicenter randomized study conducted 1998‐2000. Vnitrní lékarství 2001;47(Suppl 1):48‐56. [PubMed] [Google Scholar]
Intragumtornchai 1999 {published data only}
- Intragumtornchai T, Lekhakula A, Dhamaprasit T, Sutchartichan P, Swasdikul D. Idarubicin versus doxorubicin in combination with cytarabine as first course induction therapy in acute myelogenous leukemia: a randomized controlled trial. Asian Pacific Journal of Allergy Immunology 1999:pp.23.
Jia 2011 {published data only}
- Jia Q, Ge X, Xu Y, Li C, Guan B. Comparison of domestic idarubicin and imported daunorubicin on the treatment of acute leukemia. Journal of Leukemia and Lymphoma 2011;20:747‐9. [Google Scholar]
Lee 2012 {published data only}
- Lee JH, Joo YD, Kim H, Bae SH, Kim MK, Zang DY, et al. A prospective randomized comparison of idarubicin and high‐dose daunorubicin in the induction chemotherapy for acute myeloid leukemia: An interim analysis. Blood 2012;120:Abstract 3628. [Google Scholar]
Mandelli 1991 {published data only}
- Mandelli F, Petti MC, Ardia A, Pietro N, Raimondo F, Ganzina F, et al. A randomised clinical trial comparing idarubicin and cytarabine to daunorubicin and cytarabine in the treatment of acute non‐lymphoid leukaemia. A multicentric study from the Italian Co‐operative Group GIMEMA. European Journal of Cancer 1991;27:750‐5. [DOI] [PubMed] [Google Scholar]
- Petti MC, Mandelli F. Idarubicin in acute leukemias: experience of the Italian Cooperative Group GIMEMA. Seminars in Oncology 1989;16(1 Suppl 2):10‐5. [PubMed] [Google Scholar]
- Rotoli B. Idarubicin as first line treatment for adult (age 55‐80) AML. conclusive results of a GIMEMA randomized trial [abstract]. Haematologica 1991, issue Suppl 4:9.
Mandelli 2009 {published data only}
- Mandelli F, Vignetti M, Suciu S, Stasi R, Petti MC, Meloni G, et al. Daunorubicin versus mitoxantrone versus idarubicin as induction and consolidation chemotherapy for adults with acute myeloid leukemia: The EORTC and GIMEMA groups study AML‐10. Journal of Clinical Oncology 2009;27:5397‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignetti M, Witte TM, Suciu S, Zittoun R, Resegotti L, Liso V. Daunorubicin (DNR) vs mitoxantrone (MTZ) vs idarubicin (IDA) administered during induction and consolidation in acute myelogenous leukemia (AML) followed by autologous or allogeneic stem transplantation (SCT): results of the EORTC‐GIMEMA. Blood 2003, issue 11 (Pt 1):175a.
Masaoka 1996 {published data only}
- Masaoka T, Ogawa M, Yamada K, Kimura K, Ohashi Y. A phase II comparative study of idarubicin plus cytarabine versus daunorubicin plus cytarabine in adult acute myeloid leukemia. Seminars in Hematology 1996;33:12‐7. [PubMed] [Google Scholar]
Ohtake 2011 {published data only}
- Ohtake S, Miyawaki S, Fujita H, Kiyoi H, Shinagawa K, Usui N, et al. Randomized clinical trial of induction therapy comparing intensified daunorubicin with idarubicin in patients with previously untreated de novo acute myeloid leukemia (JALSG AML201 study) [Abstract No. 2000]. Blood 2006, issue 11 Part 1:566. [DOI] [PubMed]
- Ohtake S, Miyawaki S, Fujita H, Kiyoi H, Shinagawa K, Usui N, et al. Randomized study of induction therapy comparing standard‐dose idarubicin with high‐dose daunorubicin in adult patients with previously untreated acute myeloid leukemia: The JALSGAML201 study. Blood 2011;117:2358‐65. [DOI] [PubMed] [Google Scholar]
Pautas 2010 {published data only}
- Pautas C, Merabet F, Thomas X, Raffoux E, Gardin C, Corm S, et al. Randomized study of intensified anthracycline doses for induction and recombinant interleukin‐2 for maintenance in patients with acute myeloid leukemia age 50 to 70 years: Results of the ALFA‐9801 study. Journal of Clinical Oncology 2010;28:808‐14. [DOI] [PubMed] [Google Scholar]
- Pautas C, Thomas X, Merabet F, Raffoux E, Bourhis JH, Botton S, et al. Randomized comparison of standard induction with daunorubicin (DNR) for 3 days vs idarubicin (IDA) for 3 or 4 days in AML pts aged 50 to 70 and of maintenance with interleukin 2. final analysis of the ALFA 9801 study [Abstract No. 162]. Blood 2007, issue 11:55a.
Pignon 1996 {published data only}
- Pignon B, Witz F, Desablens B, Leprise PY, Francois S, Linassier C, et al. Treatment of acute myelogenous leukaemia in patients aged 50‐65: Idarubicin is more effective than zorubicin for remission induction and prolonged disease‐free survival can be obtained using a unique consolidation course. British Journal of Haematology 1996;94:333‐41. [DOI] [PubMed] [Google Scholar]
Récher 2014 {published data only}
- Chevallier P, Fornecker L, Lioure B, Bene MC, Pigneux A, Recher C, et al. Tandem versus single autologous peripheral blood stem cell transplantation as post‐remission therapy in adult acute myeloid leukemia patients under 60 in first complete remission: Results of the multicenter prospective phase III GOELAMS LAM‐2001 trial. Leukemia 2010;24:1380‐5. [DOI] [PubMed] [Google Scholar]
- Récher C, Béné MC, Lioure B, Pigneux A, Vey N, Delaunay J, et al. Long‐term results of a randomized phase 3 trial comparing idarubicin and daunorubicin in younger patients with acute myeloid leukaemia. Leukemia 2014;28(2):440‐3. [DOI] [PubMed] [Google Scholar]
Reiffers 1996 {published data only}
- Reiffers J, Huguet F, Stoppa AM, Molina L, Marit G, Attal M, et al. A prospective randomized trial of idarubicin vs daunorubicin in combination chemotherapy for acute myelogenous leukemia of the age group 55 to 75. Leukemia 1996;10:389‐95. [PubMed] [Google Scholar]
- Reiffers J, Riga‐Huguet F, Stoppa AM, Michallet M, Marit G, Attal M, et al. Idarubicin versus daunorubicin as treatment of acute myeloid leukemia in elderly patients. Haematologica 1991:8.
- Reiffers J, Rigal‐Huguet F, Stoppa AM, Michallet M, Marit G, Attal M, et al. A prospective study comparing idarubicin and daunorubicin in elderly patients with acute myeloid leukemia (AML). Annals of Hematology 1991:a2.
Rowe 2004 {published data only}
- Rowe JM, Neuberg D, Friedenberg W, Bennett JM, Paietta E, Makary A, et al. A phase III study of daunorubicin vs idarubicin vs mitoxantrone for older adult patients (>55 years) with acute myelogenous leukemia (AML): A study of the Eastern Co‐operative Oncology Group (E3993). Blood 1998, issue 10 Suppl 1 (Pt 1):313a.
- Rowe JM, Neuberg D, Friedenberg W, Bennett JM, Paietta E, Makary AZ, et al. A phase 3 study of three induction regimens and of priming with GM‐CSF in older adults with acute myeloid leukemia: A trial by the Eastern Cooperative Oncology Group. Blood 2004;103:479‐85. [DOI] [PubMed] [Google Scholar]
Vogler 1992 {published data only}
- Vogler WR, Velez‐Garcia E, Omura G, Raney M. A phase III trial comparing daunorubicin and idarubicin in combination with cytosine arabinoside as induction therapy for acute myelogenous leukemia [abstract]. Blood 1987, issue 5 Suppl 1:240a. [PubMed]
- Vogler WR, Velez‐Garcia E, Omura G, Raney M. A phase‐three trial comparing daunorubicin or idarubicin combined with cytosine arabinoside in acute myelogenous leukemia. Seminars in Oncology 1989;16(1 SUPPL. 2):21‐4. [PubMed] [Google Scholar]
- Vogler WR, Velez‐Garcia E, Weiner RS, Flaum MA, Bartolucci A, Omura GA, et al. A phase III trial comparing idarubicin and daunorubicin in combination with cytosine arabinoside in acute myelogenous leukemia [abstract]. Haematologica 1991, issue Suppl 4:9.
- Vogler WR, Velez‐Garcia E, Weiner RS, Floum MA, Bartolucci AA, Omura GA, et al. A phase III trial comparing idarubicin and daunorubicin in combination with cytarabine in acute myelogenous leukemia: A Southeastern Cancer Study Group study. Journal of Clinical Oncology 1992;10:1103‐11. [DOI] [PubMed] [Google Scholar]
Wang 2011 {published data only}
- Wang WJ, Sun AN, Chou HY, Li WY. IA regimen compared with MA regimen for induction therapy of newly diagnosed acute myeloid leukemia. Chinese Journal of Hematology 2011;32(12):869‐70. [Google Scholar]
Wiernik 1992 {published data only}
- Wiernik PH, Banks PL, Case DC, Arlin ZA. Cytarabine + idarubicin or daunorubicin for adults with previously untreated acute myeloid leukemia (AML) [abstract]. Haematologica 1991, issue Suppl 4:9.
- Wiernik PH, Banks PLC, Case Jr DC, Arlin ZA, Periman PO, Todd MB, et al. Cytarabine plus idarubicin or daunorubicin as induction and consolidation therapy for previously untreated adult patients with acute myeloid leukemia. Blood 1992;79:313‐9. [PubMed] [Google Scholar]
- Wiernik PH, Case DC Jr, Periman PO, Arlin ZA, Weitberg AB, Ritch PS. A multicenter trial of cytarabine plus idarubicin or daunorubicin as induction therapy for adult nonlymphocytic leukemia. Seminars in Oncology 1989;16(1 Suppl 2):25‐9. [PubMed] [Google Scholar]
References to studies excluded from this review
Belhabri 1999 {published data only}
- Belhabri A, Thomas X, Troncy J, Assouline D, Michallet M, Wattel E, et al. Continuous‐infusion carboplatin in combination with idarubicin or mitoxantrone for high‐risk acute myeloid leukemia: A randomised phase II study. Leukemia and Lymphoma 1999;36(1‐2):45‐55. [DOI] [PubMed] [Google Scholar]
Buchner 2012 {published data only}
- Buchner T, Schlenk RF, Schaich M, Dohner K, Krahl R, Krauter J, et al. Acute Myeloid Leukemia (AML): Different treatment strategies versus a common standard arm ‐ Combined prospective analysis by the German AML Intergroup. Journal of Clinical Oncology 2012;30(29):3604‐10. [DOI] [PubMed] [Google Scholar]
Candoni 2009 {published data only}
- Candoni A, Simeone E, Tiribelli M, Malagola M, Russo D, Fanin R. FLAIE (fludarabine, cytarabine, idarubicin, and etoposide), a four drug induction chemotherapy for adult acute myeloid leukemia: A single center experience. American Journal of Hematology 2009;84(10):690‐2. [DOI] [PubMed] [Google Scholar]
Castaigne 2004 {published data only}
- Castaigne S, Chevret S, Archimbaud E, Fenaux P, Bordessoule D, Tilly H, et al. Randomized comparison of double induction and timed‐sequential induction to a "3 + 7" induction in adults with AML: Long‐term analysis of the Acute Leukemia French Association (ALFA) 9000 study. Blood 2004;104(8):2467‐74. [DOI] [PubMed] [Google Scholar]
Chan‐Lam 1992 {published data only}
- Chan‐Lam D, Copplestone JA, Prentice A, Price R, Johnson S, Phillips M. Idarubicin cardiotoxicity in acute myeloid leukaemia. Lancet 1992;340(8812):185‐6. [DOI] [PubMed] [Google Scholar]
Creutzig 2000 {published data only}
- Creutzig U, Korholz D, Niemeyer CM, Kabisch K, Graf N, Reiter A, et al. 3 x 14 mg/m² idarubicin during induction: results of a pilot study in children with AML. Leukemia 2000;14(2):340‐2. [DOI] [PubMed] [Google Scholar]
Creutzig 2005 {published data only}
- Creutzig U, Zimmermann M, Ritter J, Reinhardt D, Hermann J, Henze G, et al. Treatment strategies and long‐term results in paediatric patients treated in four consecutive AML‐BFM trials. Leukemia 2005;19(12):2030‐42. [DOI] [PubMed] [Google Scholar]
Dluzniewska 2005 {published data only}
- Dluzniewska A, Balwierz W, Armata J, Balcerska A, Chybicka A, Kowalczyk J, et al. Twenty years of Polish experience with three consecutive protocols for treatment of childhood acute myelogenous leukemia. Leukemia 2005;19(12):2117‐24. [DOI] [PubMed] [Google Scholar]
Gardin 2013 {published data only}
- Gardin C, Chevret S, Pautas C, Turlure P, Raffoux E, Thomas X, et al. Superior long‐term outcome with idarubicin compared with high‐dose daunorubicin in patients with acute myeloid leukemia age 50 years and older. Journal of Clinical Oncology 2013;31(3):321‐7. [DOI] [PubMed] [Google Scholar]
Keldsen 1990 {published data only}
- Keldsen N, Karle H, Hansen NE, Nissen NI. Oral idarubicin in elderly patients with acute myeloid leukemia. European Journal of Haematology 1990;45:60. [DOI] [PubMed] [Google Scholar]
Lambertenghi‐Deliliers 1989 {published data only}
- Lambertenghi‐Deliliers G, Annaloro C, Cortelezzi A, Cortellaro M, Della Volpe A, Maiolo AT, et al. Idarubicin plus cytarabine as first‐line treatment of acute nonlymphoblastic leukemia. Seminars in Oncology 1989;16(1 Suppl 2):16‐20. [PubMed] [Google Scholar]
Lange 2008 {published data only}
- Lange BJ, Smith FO, Feusner J, Barnard DR, Dinndorf P, Feig S, et al. Outcomes in CCG‐2961, a Children's Oncology Group Phase 3 Trial for untreated pediatric acute myeloid leukemia: A report from the Children's Oncology Group. Blood 2008;111(3):1044‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leone 1999 {published data only}
- Leone G, Sica S, Pagano L. Idarubicin including regimens in acute myelogenous leukemia in elderly patients. Critical Reviews in Oncology/Hematology 1999;32(1):59‐68. [DOI] [PubMed] [Google Scholar]
Li 2013a {published data only}
- Li XY, Wang X, Li Y, Feng LL, Zhou H, Shan NN, et al. Analysis on clinical response of IA and DA regimens in the treatment of 74 newly diagnosed acute myeloid leukemia patients. Chinese Journal of Hematology 2013;34(1):67‐8. [PubMed] [Google Scholar]
Liu 2005 {published data only}
- Liu Y, Ke XY, Ma J, Shen ZX, Zhang XH, Du X, et al. Multi‐institutional randomized controlled clinical trial on China made 4‐demethoxydaunokrubicin (IDA) in the treatment of acute leukemia. Zhonghua zhong liu za zhi [Chinese Journal of Oncology] 2005;27(12):750‐2. [PubMed] [Google Scholar]
Morita 2010 {published data only}
- Morita Y, Kanamaru A, Miyazaki Y, Imanishi D, Yagasaki F, Tanimoto F, et al. Comparative analysis of remission induction therapy for high‐risk MDS and AML progressed from MDS in the MDS200 study of Japan Adult Leukemia Study Group. International Journal of Hematology 2010;91(1):97‐103. [DOI] [PubMed] [Google Scholar]
O'Brien 2002 {published data only}
- O'Brien TA, Russell SJ, Vowels MR, Oswald CM, Tiedemann K, Shaw PJ, et al. Results of consecutive trials for children newly diagnosed with acute myeloid leukemia from the Australian and New Zealand Children's Cancer Study Group. Blood 2002;100(8):2708‐16. [DOI] [PubMed] [Google Scholar]
Oriol 2003 {published data only}
- Oriol A, Ribera JM, Brunet S, Esteve J, Bueno J, Llorente A. Oral induction and consolidation chemotherapy with idarubicin and etoposide in elderly patients with acute myeloid leukemia. Haematologica 2003;88(2):229‐30. [PubMed] [Google Scholar]
Pashko 1991 {published data only}
- Pashko S, Jacobs J, Santorsa J. The cost‐effectiveness of idarubicin/cytosine arabinoside versus daunorubicin/cytosine arabinoside in the treatment of adults with acute myeloid leukemia. Clinical Therapeutics 1991;13(3):353‐60. [PubMed] [Google Scholar]
Reinhardt 2005 {published data only}
- Reinhardt D, Diekamp S, Langebrake C, Ritter J, Stary J, Dworzak M, et al. Acute megakaryoblastic leukemia in children and adolescents, excluding Down's syndrome: improved outcome with intensified induction treatment. Leukemia 2005;19(8):1495‐6. [DOI] [PubMed] [Google Scholar]
Shi 2013 {published data only}
- Shi PC, Zha J, Guo XT, Chen FL, Fan ZP, Huang F, et al. Idarubicin is superior to daunorubicin in remission induction of de novo acute myeloid leukemia patients with high MDR1 expression. Pharmacogenomics 2013;14(1):17‐23. [DOI] [PubMed] [Google Scholar]
Volkova 1993 {published data only}
- Volkova MA, Kaletin GI, Pirogova NA. The comparative evaluation of the efficacy of different therapeutic plans in acute nonlymphocytic leukemias. Terapevticheskiǐ Arkhiv 1993;65(7):18‐23. [PubMed] [Google Scholar]
Wheatley 2001 {published data only}
- Wheatley K, Hills RK. Inappropriate reporting and interpretation of subgroups in the AML‐BFM 93 study. Leukemia 2001;15(11):1803‐4. [DOI] [PubMed] [Google Scholar]
Witz 1995 {published data only}
- Witz F, Witz B, Dorvaux V, Paitel JF, Stockemer V, Bordigoni P, et al. Treatment of acute myeloid leukemia in adults. Results on 64 patients treated with the GOELAM 1 regimen. Annales Medicales de Nancy et de l'Est 1995;34(3):157‐9. [Google Scholar]
Xia 2013 {published data only}
- Xia WL, Zhang CJ, Chen XT. Treatment of IDA on elderly patients with acute myelogenous leukemia. China Medicine and Pharmacy 2013;3(12):71‐2. [Google Scholar]
References to ongoing studies
C‐022 {published data only}
- Cooperative Study Group A for Hematology. A Prospective Randomized Comparison of Idarubicin and High‐dose Daunorubicin in Combination With Cytarabine in the Induction Chemotherapy for Acute Myeloid Leukemia. www.clinicaltrials.gov/ (accessed May 19, 2010).
Additional references
AML Collaborative Group 1998
- AML Collaborative Group. A systematic collaborative overview of randomised trials comparing idarubicin with daunorubicin (or other anthracyclines) as induction therapy for acute myeloid leukaemia. British Journal of Haematology 1998;103(1):100‐9. [PubMed] [Google Scholar]
Arcamone 1976
- Arcamone F, Bernardi L, Giardino P, Patelli B, Marco A, Casazza AM, et al. Synthesis and antitumor activity of 4‐demethoxydaunorubicin, 4‐demethoxy‐7,9‐diepidaunorubicin, and their beta anomers. Cancer Treatment Reports 1976;60(7):829‐34. [PubMed] [Google Scholar]
Avvisati 1996
- Avvisati G, Lo Coco F, Diverio D, Falda M, Ferrara F, Lazzarino M, et al. AIDA (all‐trans retinoic acid + idarubicin) in newly diagnosed acute promyelocytic leukemia: a Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto (GIMEMA) pilot study. Blood 1996;88(4):1390‐8. [PubMed] [Google Scholar]
Baer 2009
- Baer MR, Greer JP. Acute myeloid leukemia in adults. In: Greer JP, Foerster J, Rodgers GM, Paraskevas F, Glader B, Arber DA editor(s). Wintrobe's Clinical Hematology. 12th Edition. Lippincott Williams & Wilkins, 2009. [Google Scholar]
Bennett 1976
- Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR. Proposals for the classification of the acute leukaemias. French‐American‐British (FAB) co‐operative group. British Journal of Haematology 1976;33(4):451‐8. [DOI] [PubMed] [Google Scholar]
Berman 1983
- Berman E, Wittes RE, Leyland‐Jones B, Casper ES, Gralla RJ, Howard J, et al. Phase I and clinical pharmacology studies of intravenous and oral administration of 4‐demethoxydaunorubicin in patients with advanced cancer. Cancer Research 1983;43(12 Pt 1):6096‐101. [PubMed] [Google Scholar]
Berman 1989
- Berman E, Raymond V, Daghestani A, Arlin ZA, Gee TS, Kempin S, et al. 4‐demethoxydaunorubicin (idarubicin) in combination with 1‐beta‐D‐arabinofuranosylcytosine in the treatment of relapsed or refractory acute leukemia. Cancer Research 1989;49(2):477‐81. [PubMed] [Google Scholar]
Berman 1992
- Berman E, McBride M. Comparative cellular pharmacology of daunorubicin and idarubicin in human multidrug‐resistant leukemia cells. Blood 1992;79(12):3267‐73. [PubMed] [Google Scholar]
Berman 1997
- Berman E, Wiernik P, Vogler R, Velez‐Garcia E, Bartolucci A, Whaley FS. Long‐term follow‐up of three randomized trials comparing idarubicin and daunorubicin as induction therapies for patients with untreated acute myeloid leukemia. Cancer 1997;80(11 Suppl):2181‐5. [DOI] [PubMed] [Google Scholar]
Burnett 2011
- Burnett A, Wetzler M, Lowenberg B. Therapeutic advances in acute myeloid leukemia. Journal of Clinical Oncology 2011;29(5):487‐94. [DOI] [PubMed] [Google Scholar]
Carella 1984
- Carella AM, Santini G, Martinengo M, Marmont AM. 4‐Demethoxydaunorubicin (idarubicin) in refractory or relapsed acute leukemias. Haematologica 1984; Vol. 69, issue 6:767‐8. [PubMed]
Carella 1987
- Carella AM, Martinengo M, Santini G, Gaozza E, Damasio E, Giordano D, et al. Idarubicin in combination with etoposide and cytarabine in adult untreated acute non lymphoblastic leukemia. European Journal of Cancer and Clinical Oncology 1987;23(11):1673‐8. [DOI] [PubMed] [Google Scholar]
Casazza 1980
- Casazza AM, Pratesi G, Giuliani F, Marco DA. Antileukemic activity of 4‐demethoxydaunorubicin in mice. Tumori 1980;66(5):549‐64. [DOI] [PubMed] [Google Scholar]
Cersosimo 1992
- Cersosimo RJ. Idarubicin: an anthracycline antineoplastic agent. Clinical Pharmacy 1992;11(2):152‐67. [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐analysis in Context. 2nd Edition. London (UK): BMJ Publishing Group, 2001. [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Deschler 2006
- Deschler B, Lubbert M. Acute myeloid leukemia: epidemiology and etiology. Cancer 2006;107(9):2099‐107. [DOI] [PubMed] [Google Scholar]
Dohner 2010
- Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010;115(3):453‐74. [DOI] [PubMed] [Google Scholar]
Douer 1996
- Douer D, Preston‐Martin S, Chang E, Nichols PW, Watkins KJ, Levine AM. High frequency of acute promyelocytic leukemia among Latinos with acute myeloid leukemia. Blood 1996;88(1):308‐13. [PubMed] [Google Scholar]
Estey 1997
- Estey E, Thall P, Kantarjian H, Pierce S, Kornblau S, Keating M. Association between increased body mass index and a diagnosis of acute promyelocytic leukemia in patients with acute myeloid leukemia. Leukemia 1997;11(10):1661‐4. [DOI] [PubMed] [Google Scholar]
Estey 2006
- Estey E, Dohner H. Acute myeloid leukaemia. Lancet 2006;368(9550):1894‐907. [DOI] [PubMed] [Google Scholar]
Fernandez 2009
- Fernandez HF, Sun Z, Yao X, Litzow MR, Luger SM, Paietta EM, et al. Anthracycline dose intensification in acute myeloid leukemia. New England Journal of Medicine 2009;361(13):1249‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ganzina 1986
- Ganzina F, Pacciarini MA, Pietro N. Idarubicin (4‐demethoxydaunorubicin). A preliminary overview of preclinical and clinical studies. Investigational New Drugs 1986;4(1):85‐105. [DOI] [PubMed] [Google Scholar]
GRADE Working Group 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2011 [Computer program]
- Jan B, Andrew O, Holger S. GRADEpro. Version 3.6 for Windows. Cochrane IMS, 2011.
Hande 2009
- Hande KR. Principles and pharmacology of chemotherapy. In: Greer JP, Foerster J, Rodgers GM, Paraskevas F, Glader B, Arber DA editor(s). Wintrobe's Clinical Hematology. 12th Edition. Lippincott Williams & Wilkins, 2009. [Google Scholar]
Harousseau 1987
- Harousseau JL, Hurteloup P, Reiffers J, Rigal‐Huguet F, Hayat M, Dufour P, et al. Idarubicin in the treatment of relapsed or refractory acute myeloid leukemia. Cancer Treatment Reports 1987; Vol. 71, issue 10:991‐2. [PubMed]
Hayat 1984
Higgins 2011a
- Higgins JPT, Deeks JJ. Chapter 7: Selecting studies and collecting data, In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jabbour 2006
- Jabbour EJ, Estey E, Kantarjian HM. Adult acute myeloid leukemia. Mayo Clinic Proceedings 2006;81(2):247‐60. [DOI] [PubMed] [Google Scholar]
Johnson 1998
- Johnson SA, Richardson DS. Anthracyclines in haematology: pharmacokinetics and clinical studies. Haematological Oncology 1998;12(1):52‐71. [DOI] [PubMed] [Google Scholar]
Kaplan 1982
- Kaplan S, Martini A, Varini M, Togni P, Cavalli F. Phase I trial of 4‐demethoxydaunorubicin with single i.v. doses. European Journal of Cancer and Clinical Oncology 1982;18(12):1303‐6. [DOI] [PubMed] [Google Scholar]
Lambertenghi‐Deliliers 1987
- Lambertenghi‐Deliliers G, Maiolo AT, Annaloro C, Cortelezzi A, Pogliani E, Ganzina F, et al. Idarubicin in sequential combination with cytosine arabinoside in the treatment of relapsed and refractory patients with acute non‐lymphoblastic leukemia. European Journal of Cancer and Clinical Oncology 1987; Vol. 23, issue 7:1041‐5. [DOI] [PubMed]
Latagliata 1997
- Latagliata R, Avvisati G, Lo Coco F, Petti MC, Diverio D, Spadea A, et al. The role of all‐trans‐retinoic acid (ATRA) treatment in newly‐diagnosed acute promyelocytic leukemia patients aged > 60 years. Annals of Oncology 1997;8(12):1273‐5. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Li 2013
- Li X, Xu SN, Tan Y, Chen JP. Idarubicin compared to other anthracyclines for treatment of newly diagnosed acute myeloid leukaemia. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD010432] [DOI] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of Clinical Epidemiology 2009;62(10):e1‐34. [DOI] [PubMed] [Google Scholar]
Little 2012
- Little RJ, D'Agostino R, Cohen ML, Dickersin K, Emerson SS, Farrar JT, et al. The prevention and treatment of missing data in clinical trials. New England Journal of Medicine 2012;367(14):1355‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lowenberg 1999
- Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. New England Journal of Medicine 1999;341(14):1051‐62. [DOI] [PubMed] [Google Scholar]
Lowenberg 2009
- Lowenberg B, Ossenkoppele GJ, Putten W, Schouten HC, Graux C, Ferrant A, et al. High‐dose daunorubicin in older patients with acute myeloid leukemia. New England Journal of Medicine 2009;361(13):1235‐48. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA Statement. Journal of Clinical Epidemiology 2009;62(10):1006‐12. [DOI] [PubMed] [Google Scholar]
NCCN 2010
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Acute Myeloid Leukemia, Version 2. 2010. Available at: http://www.nccn.org/.
NCCN 2011
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Acute Myeloid Leukemia, Version 2. 2011. Available at: http://www.nccn.org/.
NCCN 2012
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Acute Myeloid Leukemia, Version 2. 2012. Available at: http://www.nccn.org/.
NCCN 2013
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Acute Myeloid Leukemia, Version 2. 2013. Available at: http://www.nccn.org/.
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre,The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre,The Cochrane Collaboration, 2014.
Robert 1992
- Robert J, Rigal‐Huguet F, Hurteloup P. Comparative pharmacokinetic study of idarubicin and daunorubicin in leukemia patients. Hematological Oncology 1992;10(2):111‐6. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Supino 1977
- Supino R, Necco A, Dasdia T, Casazza AM, Marco A. Relationship between effects on nucleic acid synthesis in cell cultures and cytotoxicity of 4‐demethoxy derivatives of daunorubicin and adriamycin. Cancer Research 1977;37(12):4523‐8. [PubMed] [Google Scholar]
Tallman 2005
- Tallman MS, Gilliland DG, Rowe JM. Drug therapy for acute myeloid leukemia. Blood 2005;106(4):1154‐63. [DOI] [PubMed] [Google Scholar]
Teuffel 2013
- Teuffel O, Leibundgut K, Lehrnbecher T, Alonzo TA, Beyene J, Sung L. Anthracyclines during induction therapy in acute myeloid leukaemia: a systematic review and meta‐analysis. British Journal of Haematology 2013;161(2):192‐203. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trial 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vardiman 2009
- Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009;114(5):937‐51. [DOI] [PubMed] [Google Scholar]
Wang 2008
- Wang ZY, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood 2008;111(5):2505‐15. [DOI] [PubMed] [Google Scholar]
Wang 2013
- Wang J, Yang YG, Zhou M, Xu JY, Zhang QG, Zhou RF, et al. Meta‐analysis of randomised clinical trials comparing idarubicin + cytarabine with daunorubicin + cytarabine as the induction chemotherapy in patients with newly diagnosed acute myeloid leukaemia. PloS One 2013;8(4):e60699. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zittoun 1995
- Zittoun RA, Mandelli F, Willemze R, Witte TD, Labar B, Resegotti L, et al. Autologous or allogeneic bone marrow transplantation compared with intensive chemotherapy in acute myelogenous leukemia. European Organization for Research and Treatment of Cancer (EORTC) and the Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto (GIMEMA) Leukemia Cooperative Groups. New England Journal of Medicine 1995; Vol. 332, issue 4:217‐23. [DOI] [PubMed]


