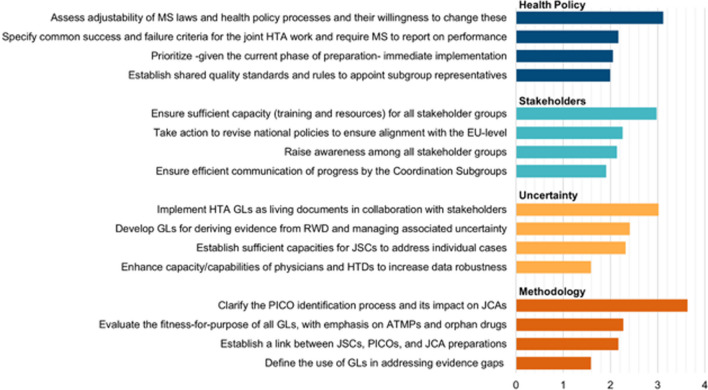
Fig. 4.
Relative importance of key action points within the area of focus for each break-out session, as prioritized by the respondents during the plenary session. Cumulative weighted responses for each list item were divided by the total votes (Health Policy: N = 40; Stakeholders: N = 43; Uncertainty: N = 41; Methodology: N = 41) to express an average of the maximum possible weight, i.e., 4 points. ATMPs, Advanced Therapy Medicinal Products; EU, European Union; GL, Guideline; HTA, Health Technology Assessment; HTD, Health Technology Developer; JCA, Joint Clinical Assessment; JSC, Joint Scientific Consultation; MS, Member States; PICO, Population, Intervention, Comparator(s), Outcomes; PLEG, Post Launch Evidence Generation; RWD, Real-World Data