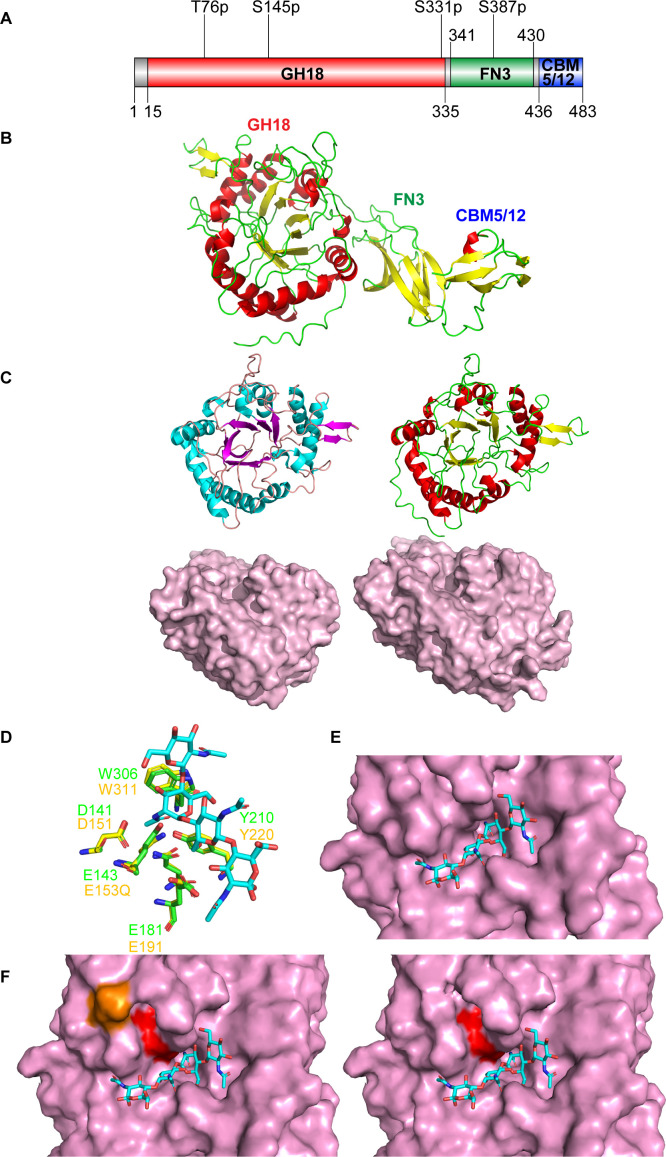
Fig 1.
ChiC sequence analysis and tertiary structure prediction. (A) Domain architecture of ChiC. The full-length protein comprises three domains: a glycoside hydrolase 18 domain (GH18), a fibronectin type III domain (FN3), and a carbohydrate binding module 5 or 12 (CBM5/12). Potential post-translational modifications (PTMs) are depicted above (27). The first character is the amino acid followed by the amino acid number and the type of PTM. All postulated PTMs are phosphorylations (p). (B) Model of ChiC predicted by AlphaFold2 provided by UNINETT Sigma2. (C) Comparison of the structures of the GH18 domains of SmChiC (PDB: 4AXN) (left) and ChiC (right). The GH18 domains are shown as both cartoons and surface models. The surface models accentuate the binding clefts of the GH18 domains. (D) Comparison of the active sites of ChiC with the chitinase of Moritella marina E153Q mutant in complex with (GlcNAc)4 (PDB ID: 4MB4). (E) Showing (GlcNAc)4 (PDB ID: 4MB4) superimposed into the active site of ChiC. (F) Comparison of the active site of the GH18 domain of ChiC with (GlcNAc)4 (PDB ID: 4MB4), with (orange) and without phosphorylated serine at position 145. The catalytic glutamate at position 143 is highlighted in red. The addition of the phosphate group to serine was done using the plugin PyTMs version 1.2 in PyMol.