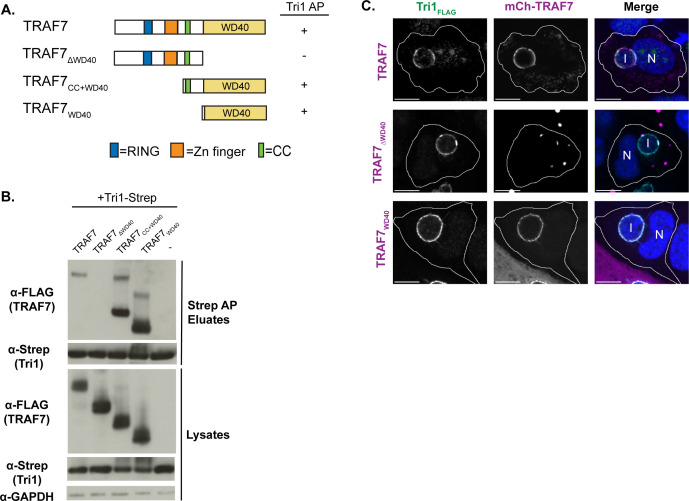
Fig 4.
The WD40 of TRAF7 is necessary and sufficient to interact with Tri1. (A) Schematic of TRAF7 constructs. Zn, zinc. CC, coiled-coil. RING, RING finger ubiquitin ligase domain. Variants that interact with TRAF7 by co-AP are indicated with a “+” sign, and variants that do not interact are indicated with a ”−“ sign. (B) Lysates and eluates of HEK293T cells co-transfected with Tri1-Strep and the indicated FLAG-TRAF7 variants (“−” indicates the control with no TRAF7 added) were affinity purified with Strep-Tactin beads and immunoblotted with the indicated antibodies. GAPDH serves as a loading control for lysates. Only variants containing the WD40 domain of TRAF7 co-affinity purified with Tri1. The slower migrating band present in some of the TRAF7 samples likely represents stable dimers. (C) Confocal immunofluorescence microscopy of HeLa cells transfected with the indicated mCh-TRAF7 variants for 24 h followed by infection L2+pTri1FLAG in the presence of aTc for 24 h. Cells were fixed and stained with α-FLAG and DAPI and imaged by confocal microscopy. Cell membranes are outlined. Tri1 is pseudocolored green and TRAF7 is pseudocolored magenta in the merged image. Shown are single z-slices. Scale bar = 10 µm. I, inclusion. N, nucleus.