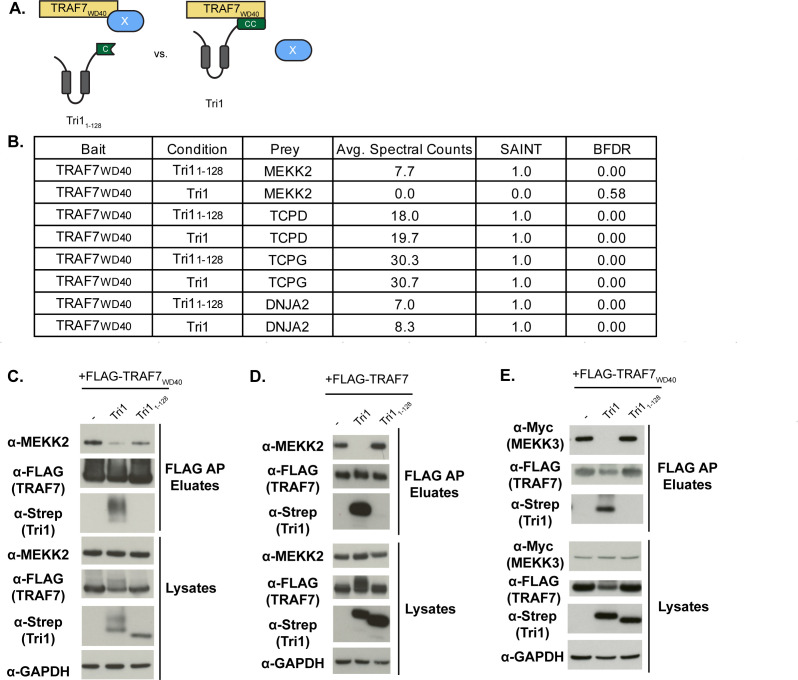
Fig 5.
Tri1 displaces MEKK2 and MEKK3 binding to TRAF7. (A) Schematic of displacement AP-MS analysis with a potential displaced TRAF7 native interactor represented by “X.” (B) HEK293T cells co-transfected with FLAG-TRAF7WD40 (bait) and either Tri11-128-Strep or Tri1-Strep. Lysates were affinity purified over FLAG beads and analyzed by LC/MS-MS. Shown are the average spectral counts from three biological replicates, SAINT scores, and BFDR of selected TRAF7WD40 interacting partners in the presence of Tri11-128-Strep or Tri1-Strep. SAINT scores closer to 1 with a BFDR ≤ 0.05 suggest a high confidence interaction. Tri1 displaces MEKK2 binding to TRAF7WD40 but not to TCPD, TCPG, or DNJA2. (C–E) Validation of Tri1-mediated displacement of MEKK2 and MEKK3 binding to the TRAF7 WD40 domain by co-transfection studies. HEK293T cells were co-transfected with either Tri1-Strep or Tri184-147- Strep (C–E), FLAG-TRAF7WD40 (C and E), FLAG-TRAF7 (D), and Myc-MEKK3 (E). Cells only transfected with FLAG-TRAF7WD40 (C and E) and FLAG-TRAF7 (D) were designated “−” and served as a control. Lysates were affinity purified using FLAG beads and analyzed by immunoblot with the indicated antibodies. GAPDH serves as a loading control for the lysates. Full-length Tri1, but not Tri11-128, disrupts TRAF7 binding to MEKK2 and to MEKK3.