Abstract
Cardiac arrhythmias significantly contribute to mortality in Duchenne muscular dystrophy (DMD), a severe muscle illness caused by mutations in the gene encoding for the intracellular protein dystrophin. A major source for arrhythmia vulnerability in patients with DMD is impaired ventricular impulse conduction, which predisposes for ventricular asynchrony, decreased cardiac output, and the development of reentrant circuits. Using the dystrophin-deficient mdx mouse model for human DMD, we previously reported that the lack of dystrophin causes a significant loss of peak Na+ current (INa) in ventricular cardiomyocytes. This finding provided a mechanistic explanation for ventricular conduction defects and concomitant arrhythmias in the dystrophic heart. In the present study, we explored the hypothesis that empagliflozin (EMPA), an inhibitor of sodium/glucose cotransporter 2 in clinical use to treat type II diabetes and nondiabetic heart failure, rescues peak INa loss in dystrophin-deficient ventricular cardiomyocytes. We found that INa of cardiomyocytes derived from mdx mice, which had received clinically relevant doses of EMPA for 4 wk, was restored to wild-type level. Moreover, incubation of isolated mdx ventricular cardiomyocytes with 1 µM EMPA for 24 h significantly increased their peak INa. This effect was independent of Na+-H+ exchanger 1 inhibition by the drug. Our findings imply that EMPA treatment can rescue abnormally reduced peak INa of dystrophin-deficient ventricular cardiomyocytes. Long-term EMPA administration may diminish arrhythmia vulnerability in patients with DMD.
NEW & NOTEWORTHY Dystrophin deficiency in cardiomyocytes leads to abnormally reduced Na+ currents. These can be rescued by long-term empagliflozin treatment.
Keywords: arrhythmias, cardiomyocyte sodium currents, Duchenne muscular dystrophy, empagliflozin, mdx mice
INTRODUCTION
Cardiac arrhythmias significantly contribute to mortality in Duchenne muscular dystrophy (DMD), a fatal disease caused by dystrophin deficiency (1, 2). Impaired ventricular impulse conduction, predisposing for ventricular asynchrony and the development of reentrant circuits, is a major source of arrhythmias in patients with DMD. Using the dystrophin-deficient mdx mouse model for human DMD (3), we and others previously reported that the lack of dystrophin causes a significant peak Na+ current (INa) loss both in ventricular cardiomyocytes of the working myocardium (4–6) and in Purkinje fibers (7, 8), ventricular myocytes specialized for rapid electrical impulse conduction. These findings provided a mechanistic explanation for ventricular conduction defects and concomitant arrhythmias in the dystrophic heart.
Empagliflozin (EMPA), an inhibitor of sodium/glucose cotransporter 2 (SGLT2) in clinical use to treat type II diabetes and as of late also nondiabetic heart failure (HF) (9, 10), was recently shown to modulate cardiac Na+ channels. Thus, application of the drug selectively inhibited the detrimental late INa, which occurs in ventricular cardiomyocytes originating from mouse models for HF (11–13). EMPA may thereby exert an antiarrhythmic effect. Dago et al. (14) recently tested the effects of 24-h incubation with EMPA at a therapeutically relevant concentration (1 µM) on INa in cardiomyocytes derived from human-induced pluripotent stem cells. These authors found that EMPA treatment significantly increases peak INa, suggesting an upregulation of Na+ channel expression under lasting presence of the drug. If EMPA would exert a similar effect in dystrophin-deficient ventricular cardiomyocytes, the drug could be used to counteract peak INa loss in the dystrophic heart.
In this study, we used the mdx mouse model for DMD to test the hypothesis that long-term treatment with EMPA rescues peak INa loss in dystrophin-deficient ventricular cardiomyocytes.
METHODS
Ethical Approval
The investigation conformed to the guiding principles of the Declaration of Helsinki and coincides with the rules of the Animal Welfare Committee of the Medical University of Vienna. The experimental protocols, which were applied during the study, were approved by the Austrian Science Ministry. The respective ethics vote has the following number: BMWFW-66.009/0175-WF/V/3b/2015.
Mice and Long-Term EMPA Treatment
Dystrophin-deficient mdx mice on the BL10 background (C57BL/10ScSn-Dmdmdx/J) and wild-type control mice (C57BL/10ScSnJ) in an age range between 16 and 23 wk were used for the experiments. These two mouse lines were originally purchased from Charles River Laboratories. Only male mice were used in this study because of the X-linked inheritance of DMD and potential translational relevance to human patients as in Haffner et al. (15). Genotyping of the mice was performed using standard PCR assays. A cohort of mdx mice received EMPA (MedChemExpress) via the drinking water. Treatment started at 12 wk of age and lasted for 4 wk. Drinking water containing EMPA was freshly prepared on a weekly basis. First, EMPA was dissolved in dimethyl sulfoxide (DMSO) to obtain a 50 mg/mL stock solution. Then, the stock solution was diluted in drinking water to achieve a final EMPA concentration of 0.1 mg/mL (0.2% DMSO). In consideration of a mean water intake of 5 mL/day and a mean body weight of 33 g, the EMPA concentration of 0.1 mg/mL in the drinking water resulted in an EMPA dose of ∼15 mg/kg body wt/day, a dose lying within the standard range for studies with mice (16–19). Administering EMPA at such a typical dose to mice was previously shown to lead to plasma concentrations comparable with therapeutic EMPA plasma concentrations in humans (19). During treatment with 15 mg/kg/day EMPA, the water intake of the mdx mice was twofold increased when compared with that of untreated control mdx mice. This confirmed that the applied EMPA dose was effective and led to SGLT2 inhibition. Mice of the control groups, which drank ∼2.5 mL/day, were exposed to drinking water comprising 0.4% of the solvent DMSO for 4 wk.
Isolation of Ventricular Cardiomyocytes and Drug Incubation Procedure
Wild-type and mdx mice were anesthetized using isoflurane (2%, inhalation) and euthanized by cervical dislocation. Cardiomyocytes were then isolated from the ventricles of their hearts using a Langendorff setup (Hugo Sachs Elektronik, March, Germany) according to the isolation procedure described in detail in our previous work (5). In brief, the hearts were rapidly excised, and a cannula was inserted into the aorta for retrograde perfusion with Ca2+-free solution containing 0.17 mg/mL Liberase TH (Roche) at 37°C for 10 min. Thereafter, the ventricles were cut into pieces and incubated on a shaker at 37°C. Subsequently, the Ca2+ concentration was increased to 150 μM over 30 min in four steps. Pieces of digested tissue were then triturated to liberate ventricular cardiomyocytes. After a centrifugation step, the myocytes were resuspended in minimum essential medium (MEM)-α (Sigma), containing ITS media supplement (Sigma) diluted (1:100), 2 mM l-glutamine, 100 U/mL penicillin, 0.1 mg/mL streptomycin, and 17 µM blebbistatin (Sigma). Myocytes were finally plated on Matrigel (Becton Dickinson)-coated culture dishes. For incubation experiments, cells were exposed to 1 µM EMPA, 10 µM cariporide (MedChemExpress), or 10 µM cariporide in combination with 1 µM EMPA for 24 h by adding these drugs from 100 mM stock solutions in DMSO to the cell culture medium. Cardiomyocytes of the control groups were incubated with DMSO alone. DMSO concentrations were equal in all compared experimental groups. Drug-treated cardiomyocytes and DMSO-treated control cells always originated from the same cardiomyocyte isolation (identical mouse).
INa Recordings
The whole cell patch clamp technique was used to record INa from ventricular cardiomyocytes up to 6 h after preparation (except for 24-h incubation experiments). The recordings were performed at room temperature (22 ± 1.5°C) by using an Axopatch 200B patch clamp amplifier (Axon Instruments, Union City, CA). Pipettes were formed from aluminosilicate glass (A120-77-10; Science Products, Hofheim, Germany) with a P-97 horizontal puller (Sutter Instruments, Novato, CA). They had resistances between 0.8 and 1.2 MΩ when filled with pipette solution. Data acquisition was performed with pClamp 10 software (Axon Instruments) through a 16-bit A-D/D-A interface (Digidata1440; Axon Instruments). Data were low-pass filtered with 10 kHz (−3 dB) and digitized at 35 kHz. Data analysis was performed with Clampfit 10.7 (Axon Instruments) and GraphPad Prism 8 (San Diego, CA) software. Current-voltage (I-V) relationships were fit with the function: I = Gmax·(V − Vrev)/{1 + exp[(V50 − V)/K]}, where I is the current, Gmax is the maximum conductance, V is the membrane potential, Vrev is the reversal potential, V50 is the voltage at which half-maximum activation occurred, and K is the slope factor. Membrane voltages were corrected for liquid junction potentials. For current density-voltage relationships, the current amplitudes at various voltages were measured. These values were then divided by the membrane capacitance to yield current densities. A holding potential of −117 mV, from which the channels were activated by depolarizing voltage steps, was chosen to guarantee full channel availability. Steady-state fast inactivation data were fit with the Boltzmann function: I/Imax = 1/(1 + exp[(V − V50)/K)], where I/Imax is the normalized current, V is the membrane potential, V50 is the voltage at which half-maximum inactivation occurred, and K is the slope factor. Recordings from ventricular cardiomyocytes were made in a bath solution that consisted of (in mM) 5 NaCl, 135 N-methyl-d-glucamine, 2.5 KCl, 1 CaCl2, 1 MgCl2, and 10 HEPES (pH 7.4), adjusted with HCl. The bath solution additionally contained 17 µM blebbistatin. The pipette solution contained (in mM) 5 NaCl, 110 CsF, 10 EGTA, and 10 HEPES (pH 7.3), adjusted with CsOH. A DAD-8-VC superfusion system (ALA Scientific Instruments, Westbury, NY) was used for continuous superfusion of patched cells and allowed for rapid extracellular solution changes.
Statistical Data Analysis
Comparisons were made using a nested analysis respecting the hierarchical data structure (measurements of n cells from m animals) detailed in Sikkel et al. (20). A P value <0.05 was considered significantly different.
RESULTS
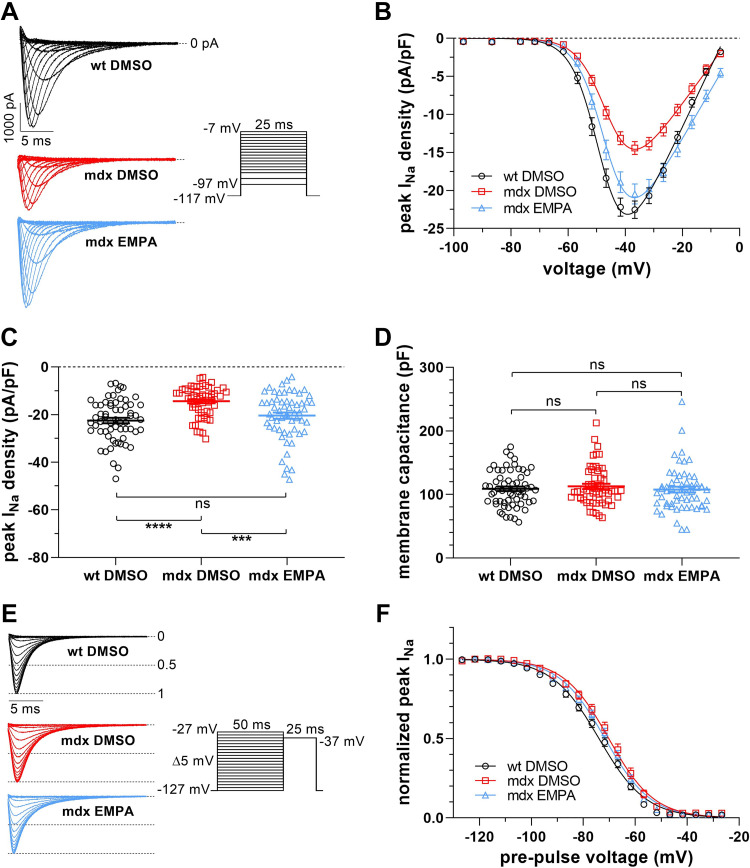
The effects of EMPA on INa of dystrophin-deficient ventricular cardiomyocytes were studied in a series of experiments. First, INa of myocytes derived from mdx mice, which had received 15 mg/kg/day EMPA via the drinking water for 4 wk, was compared with INa of myocytes from control mdx mice. Figure 1, A and B, shows that the peak INa density of EMPA-exposed mdx myocytes was markedly increased over a wide voltage range and comparable to the peak INa density of wild-type myocytes. At −37 mV, the voltage at which the INa density was maximal, a highly significant difference existed between EMPA-exposed mdx and control mdx cardiomyocytes (Fig. 1C). The comparison between EMPA-exposed mdx and wild-type myocytes revealed no significant difference (P = 0.19). These experiments suggested that, by long-term EMPA treatment, abnormally reduced INa of ventricular cardiomyocytes from dystrophin-deficient mdx mice can be rescued. Furthermore, Fig. 1, E and F, shows that the voltage dependence of INa steady-state fast inactivation was similar in wild-type, mdx, and EMPA-exposed mdx myocytes. The same was true for the voltage dependence of INa activation (for respective parameters of INa activation and inactivation see Table 1).
Figure 1.
Na+ current (INa) properties in ventricular cardiomyocytes derived from wild-type (WT DMSO), untreated control mdx (mdx DMSO) and EMPA-treated mdx (mdx EMPA) mice. The latter mdx mouse cohort had received 15 mg/kg/day EMPA via the drinking water for 4 wk; WT and control mdx mice had received a respective concentration of DMSO. A: typical original current traces of a WT, a control mdx, and an EMPA-treated mdx cardiomyocyte, elicited by the pulse protocol displayed (inset). B: from a series of such experiments [n = 61 cells from 4 WT hearts (black); n = 56 cells from 4 control mdx hearts (red); n = 58 cells from 4 mdx EMPA hearts (light blue)], current density-voltage relationships were derived. Data points are represented as means ± SE. Parameters for INa activation derived from fits of the current-voltage relationships (function described in methods) are given in Table 1. C: dot plot comparing the maximum peak INa densities of WT, control mdx, and EMPA-treated mdx cardiomyocytes at −37 mV. ****P < 0.0001, significant difference between WT and mdx. ***P < 0.001, significant difference between mdx and mdx EMPA. ns, not significant (P = 0.19). D: dot plot comparing membrane capacitance values for cell size estimation of WT, control mdx, and EMPA-treated mdx cardiomyocytes. ns, not significant (P always > 0.38). E: original INa traces of a WT, a control mdx, and an EMPA-treated mdx cardiomyocyte, elicited by a 25-ms test pulse following a series of inactivating 50-ms prepulses. The pulse protocol used to test the voltage dependence of steady-state fast inactivation is displayed (inset). F: voltage dependencies of steady-state inactivation in WT, control mdx, and mdx EMPA cardiomyocytes (n = 48 cells from 4 WT hearts; n = 31 cells from 4 control mdx hearts; n = 32 cells from 4 mdx EMPA hearts). Parameters for steady-state fast inactivation derived from fits with a Boltzmann function (see methods) are given in Table 1.
Table 1.
Parameters of Na+ current activation and fast inactivation in ventricular cardiomyocytes
| Activation |
Inactivation |
||||||
|---|---|---|---|---|---|---|---|
| V50, mV | K, mV | Vrev, mV | n | V50, mV | K, mV | n | |
| In vivo EMPA treatment (corresponding to Fig. 1) | |||||||
| WT DMSO | −48.04 ± 0.53 | 4.05 ± 0.08 | −4.9 ± 0.66** | 61 | −74.07 ± 0.99 | −8.12 ± 0.16 | 48 |
| mdx DMSO | −45.86 ± 0.64 | 3.99 ± 0.08 | −3.12 ± 0.95* | 56 | −70.39 ± 1.26 | −7.75 ± 0.24 | 31 |
| mdx EMPA | −45.65 ± 0.61 | 4.19 ± 0.08 | 0.85 ± 0.97 | 58 | −71.6 ± 1.01 | −8.11 ± 0.19 | 32 |
| EMPA incubation of cardiomyocytes (corresponding to Fig. 2) | |||||||
| mdx DMSO | −46 ± 0.86 | 4.28 ± 0.1 | −1.54 ± 1.14 | 36 | −75.35 ± 1.91 | −7.73 ± 0.33 | 15 |
| mdx EMPA | −47.76 ± 0.79 | 4.01 ± 0.11 | 1.78 ± 1.32 | 43 | −73.94 ± 2.02 | −7.7 ± 0.18 | 18 |
Values are means ± SE; n, number of cells. Current-voltage (I-V) relationships were fit with the function: I = Gmax·(V − Vrev)/{1 + exp[(V50 − V)/K]}, where I is the current, Gmax is the maximum conductance, V is the membrane potential, Vrev is the reversal potential, V50 is the voltage at which half-maximum activation occurred, and K is the slope factor. **P < 0.01, significant difference between WT DMSO and mdx EMPA. *P < 0.05, significant difference between mdx DMSO and mdx EMPA. Steady-state fast inactivation data were fit with the Boltzmann function: I/Imax = 1/{1 + exp[(V − V50)/K]}, where I/Imax is the normalized current. EMPA, empagliflozin; WT, wild-type.
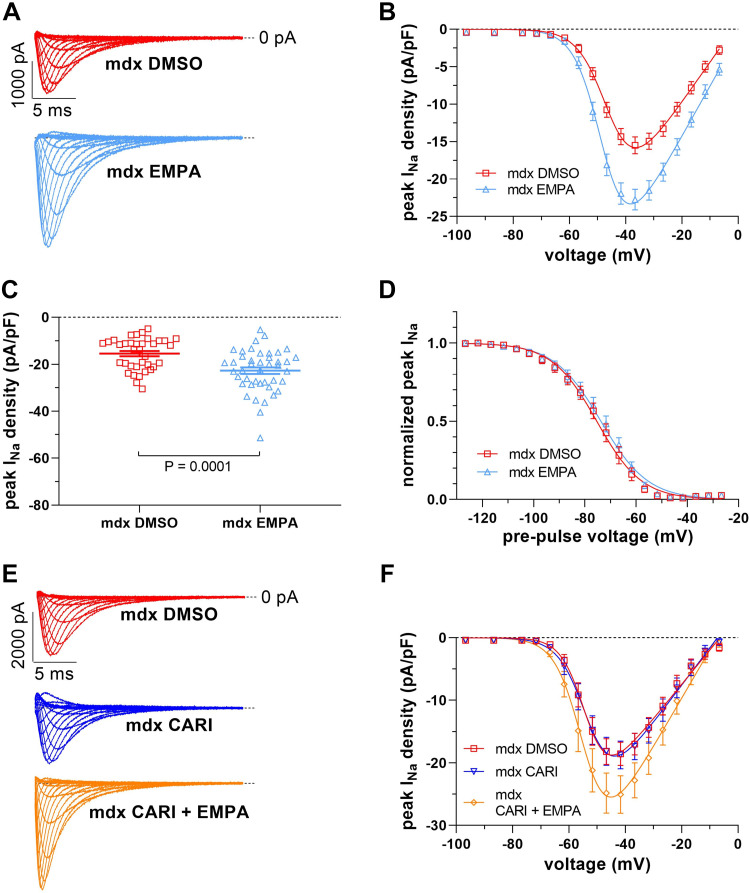
We next incubated ventricular cardiomyocytes isolated from mdx mice with 1 µM EMPA for 24 h. Thereafter, their INa was measured and compared with INa of control mdx myocytes from the same preparation. Figure 2, A–C, shows that EMPA incubation significantly increased the peak INa density of mdx myocytes to a similar extent as in the mdx mouse EMPA treatment experiment described earlier. Again, the voltage dependencies of INa activation (Table 1) and steady-state fast inactivation (Fig. 2D and Table 1) were not affected by the presence of EMPA. One of the potential targets of EMPA in the heart is the Na+-H+ exchanger 1 (NHE-1), which is known to be inhibited by the drug (21–23). To test the potential involvement of NHE-1 in the observed EMPA effect on INa of mdx ventricular cardiomyocytes, we used the selective NHE-1 inhibitor cariporide. Figure 2, E and F, shows that incubation of mdx myocytes with 10 µM cariporide (23, 24) for 24 h had no effect on the peak INa density. Furthermore, the presence of cariporide during EMPA incubation did not affect EMPA’s enhancing effect on the peak INa density. These experiments suggested that EMPA likely acts via another mechanism than NHE-1 inhibition.
Figure 2.
Effect of 24-h incubation with 1 µM empagliflozin (EMPA) on Na+ current (INa) properties of ventricular cardiomyocytes derived from mdx mice. A: typical original current traces of a control mdx (mdx DMSO) and an EMPA-treated (mdx EMPA) cardiomyocyte (for pulse protocol see Fig. 1A). B: from a series of such experiments [n = 36 mdx cells (red); n = 43 mdx EMPA cells (light blue); all cells originating from 4 mdx hearts], current density-voltage relationships were derived. C: dot plot comparing the maximum peak INa densities of control mdx and EMPA-treated mdx cardiomyocytes at −37 mV. P = 0.0001, significant difference between mdx and mdx EMPA. D: voltage dependencies of steady-state inactivation (for pulse protocol, see Fig. 1E) in control mdx and mdx EMPA cardiomyocytes (n = 15 mdx cells; n = 18 mdx EMPA cells; all cells originating from 4 mdx hearts). INa activation and steady-state fast inactivation parameters are given in Table 1. E: original current traces of a control mdx (mdx DMSO), a cariporide-treated mdx (mdx CARI, 24-h incubation at 10 µM concentration), and a cariporide-plus EMPA-treated (mdx CARI + EMPA) cardiomyocyte. F: current density-voltage relationships derived from a series of such experiments [n = 16 mdx cells (red); n = 19 mdx CARI cells (dark blue); n = 15 mdx CARI + EMPA cells (orange); all cells originating from 2 mdx hearts].
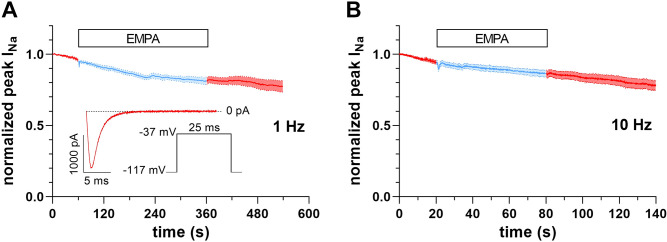
In a final set of experiments, we tested the acute effects of EMPA on peak INa of mdx ventricular cardiomyocytes. Figure 3, A and B, shows that superfusion of mdx myocytes with bath solution containing 1 µM EMPA did not affect peak INa. Acute application of 10 µM EMPA also did not impact peak INa (data not shown). These experiments suggested that EMPA does not acutely modulate peak INa of dystrophin-deficient ventricular cardiomyocytes.
Figure 3.
Lack of acute empagliflozin (EMPA) effects on peak Na+ current (INa) of mdx ventricular cardiomyocytes. A: peak INa before, during superfusion with bath solution containing 1 µM EMPA (light blue), and after washout over time (n = 13 cells from 2 mdx hearts). Data are expressed as means ± SE. Inset: pulse protocol, applied at 1-Hz frequency and elicited INa at the very beginning of the experiment. B: equivalent experiment as described in A at 10-Hz frequency (n = 15 cells from 2 mdx hearts).
DISCUSSION
Here, we report that ventricular cardiomyocytes derived from dystrophin-deficient mdx mice, which had received 15 mg/kg/day EMPA via the drinking water for 4 wk, showed peak INa densities comparable with myocytes from wild-type mice. Furthermore, 24-h incubation of isolated mdx ventricular cardiomyocytes with 1 µM EMPA significantly enhanced their INa. EMPA treatment studies with mice are typically conducted in a dose range between 10 and 35 mg/kg body wt/day (16–19). These doses were shown to lead to therapeutic plasma concentrations (19), and 1 µM EMPA is considered a therapeutically relevant concentration (11, 12, 14). Consequently, our findings suggest that long-term treatment with therapeutic concentrations of EMPA rescues peak INa loss in dystrophin-deficient ventricular cardiomyocytes.
Potential Mechanism(s) of Action
In our hands, EMPA did not exert acute effects on peak INa of dystrophin-deficient ventricular cardiomyocytes (Fig. 3). This agrees with Philippaert et al. (11), who reported that acutely applied EMPA had only little effect on peak INa of cardiomyocytes from mice with HF. Interestingly, in contrast to peak INa, late INa was significantly inhibited by acute EMPA application, and these authors proposed a direct interaction of the drug with the Nav1.5 Na+ channel. Other authors, however, did not observe acute EMPA effects on late INa of HF cardiomyocytes, but reported that the drug inhibited late INa only after a prolonged incubation period (12). Rather than a direct interaction of EMPA with Nav1.5, this suggested an indirect mechanism, possibly via inhibition of Ca2+/calmodulin-dependent protein kinase-II (CaMKII) by the drug (12, 13). Future studies should clarify this issue.
Increased peak INa of dystrophin-deficient ventricular cardiomyocytes under sustained presence of EMPA without considerably altered channel gating (Figs. 1 and 2; Table 1) suggests drug-induced upregulation of Nav1.5 channel protein expression. Enhancement of Nav1.5 expression by EMPA in the dystrophic heart accords with significantly increased Scn5a gene transcript levels observed in cardiac samples from SGLT2 inhibitor-treated HF mouse models (25, 26). With regard to EMPA’s potential mode(s) of action, we can only speculate. Since SGLT2 is not or hardly expressed in cardiac tissue (27–29), EMPA must exert its effects on Nav1.5 expression in dystrophin-deficient cardiomyocytes via another mechanism than SGLT2 inhibition. A potential alternative target is NHE-1, which is known to be inhibited by EMPA (21–23). Here, we show that incubation with cariporide, a selective NHE-1 inhibitor, had no effect on peak INa of dystrophin-deficient cardiomyocytes, and that the presence of cariporide during EMPA incubation did not affect EMPA’s impact on peak INa (Fig. 2, E and F). This suggests that inhibition of NHE-1 by EMPA can be excluded as the underlying mechanism under our experimental conditions. Another conceivable mode of action of EMPA is modulation of intracellular Ca2+ handling with secondary impact on Na+ channel expression. Several research laboratories previously reported that EMPA and other SGLT2 inhibitors significantly improve impaired Ca2+ handling properties in HF cardiomyocytes (23, 27, 30–32). Among these improvements, a reduction in diastolic (resting) Ca2+ in the presence of a SGLT2 inhibitor was observed (31). A decrease in resting Ca2+ was also found in isolated healthy murine cardiomyocytes after an EMPA exposure for 24 h (30). Diminished resting Ca2+ levels are known to induce an upregulation of Na+ channel expression in myocytes (33, 34). We therefore speculate that EMPA-induced upregulation of Nav1.5 expression in dystrophin-deficient ventricular cardiomyocytes may result from reduced resting Ca2+ levels in the presence of the drug. EMPA’s actual mode of action remains to be elucidated.
Study Limitations and Future Directions
Although we assume that the enhancement of peak INa of dystrophin-deficient ventricular cardiomyocytes due to EMPA treatment results from increased Nav1.5 channel expression, we have not confirmed this experimentally. Alternative explanations would be drug-induced upregulation of noncardiac Na+ channel isoforms, alterations in Na+ channel localization, or enhancement of channel conductance. Furthermore, we encourage researchers to test a potential involvement of CaMKII, which reduces peak INa of cardiomyocytes (35). EMPA inhibits CaMKII (12, 13) and may thereby have an enhancing effect on peak INa. In the present study, we have not tested EMPA effects on peak INa of control ventricular cardiomyocytes from wild-type mice. Significantly increased peak INa of cardiomyocytes derived from human-induced pluripotent stem cells after EMPA incubation (14), however, suggests that the drug’s effect does not only occur in case of dystrophin deficiency. The effect of EMPA on late INa in dystrophin-deficient ventricular cardiomyocytes was also not studied here, because there is no evidence in the literature for the occurrence of enhanced late INa in dystrophin-deficient versus wild-type myocytes. Furthermore, EMPA effects on peak INa of dystrophin-deficient cardiac Purkinje fibers (7, 8) should be tested, because this cell type is a major determinant of ventricular conduction velocity. Finally, we encourage a comparison of the arrhythmia incidence in EMPA-treated versus untreated mdx mice.
Clinical Relevance
SGLT2 inhibitors have recently been approved for the treatment of patients with HF but not diabetes. Among various potential beneficial effects for the diseased heart, these drugs are antiarrhythmic, whereby inhibition of late INa of HF cardiomyocytes is considered a relevant mechanism (11–13). To the best of our knowledge, the effectiveness of SGLT2 inhibitors in DMD cardiomyopathy is unexplored. Significantly reduced peak INa, a characteristic feature of dystrophin-deficient ventricular cardiomyocytes (4–8), represents a relevant source of cardiac arrhythmias. Accordingly, Scn5a+/− mice (animal model for the Brugada syndrome) (36, 37), which have a similarly reduced cardiomyocyte peak INa as mdx mice (4–6), show ventricular arrhythmias and conduction disorders (36). Here, we report that EMPA treatment of mdx mice completely rescues peak INa loss in dystrophin-deficient ventricular cardiomyocytes. Thereby, we have corrected the source of impaired ventricular conduction and concomitant arrhythmias in the dystrophic heart. We speculate that EMPA treatment may improve ventricular conduction and prevent arrhythmias in human patients with DMD. EMPA may also be useful for the treatment of other arrhythmic disorders triggered by Na+ channel loss of function.
Collectively, our study implies that EMPA treatment can rescue abnormally reduced peak INa of dystrophin-deficient ventricular cardiomyocytes. Further studies are needed to explore whether this effect is EMPA specific or common to SGLT2 inhibitors in general, and whether EMPA administration can diminish arrhythmia vulnerability in patients with DMD.
DATA AVAILABILITY
The data underlying this article will be shared on reasonable request to the corresponding author.
GRANTS
This work was supported by Austrian Science Fund (FWF) Grants P35542-B and P35878-B (to K. Hilber).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.S., A.K., and K.H. conceived and designed research; J.S., J.M., E.L., and B.H. performed experiments; J.S., J.M., B.H., and C.D. analyzed data; J.S., H.T., H.K., B.K.P., A.K., X.K., and K.H. interpreted results of experiments; J.S., E.L., and C.D. prepared figures; J.S., X.K., and K.H. drafted manuscript; H.T., H.K., B.K.P., A.K. X.K., and K.H. edited and revised manuscript; J.S., J.M., E.L., B.H., H.T., H.K., C.D., B.K.P., A.K., X.K., and K.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge J. Uhrinova (Medical University of Vienna) for excellent technical assistance.
REFERENCES
- 1. Kamdar F, Garry DJ. Dystrophin-deficient cardiomyopathy. J Am Coll Cardiol 67: 2533–2546, 2016. doi: 10.1016/j.jacc.2016.02.081. [DOI] [PubMed] [Google Scholar]
- 2. Duan D, Goemans N, Takeda S, Mercuri E, Aartsma-Rus A. Duchenne muscular dystrophy. Nat Rev Dis Primers 7: 13, 2021. doi: 10.1038/s41572-021-00248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bulfield G, Siller WG, Wight PA, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA 81: 1189–1192, 1984. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gavillet B, Rougier JS, Domenighetti AA, Behar R, Boixel C, Ruchat P, Lehr HA, Pedrazzini T, Abriel H. Cardiac sodium channel Nav1.5 is regulated by a multiprotein complex composed of syntrophins and dystrophin. Circ Res 99: 407–414, 2006. doi: 10.1161/01.RES.0000237466.13252.5e. [DOI] [PubMed] [Google Scholar]
- 5. Koenig X, Dysek S, Kimbacher S, Mike AK, Cervenka R, Lukacs P, Nagl K, Dang XB, Todt H, Bittner RE, Hilber K. Voltage-gated ion channel dysfunction precedes cardiomyopathy development in the dystrophic heart. PLoS One 6: e20300, 2011. doi: 10.1371/journal.pone.0020300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marchal GA, van Putten M, Verkerk AO, Casini S, Putker K, van Amersfoorth SCM, Aartsma-Rus A, Lodder EM, Remme CA. Low human dystrophin levels prevent cardiac electrophysiological and structural remodelling in a Duchenne mouse model. Sci Rep 11: 9779, 2021. doi: 10.1038/s41598-021-89208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ebner J, Uhrin P, Szabo PL, Kiss A, Podesser BK, Todt H, Hilber K, Koenig X. Reduced Na+ current in Purkinje fibers explains cardiac conduction defects and arrhythmias in Duchenne muscular dystrophy. Am J Physiol Heart Circ Physiol 318: H1436–H1440, 2020. doi: 10.1152/ajpheart.00224.2020. [DOI] [PubMed] [Google Scholar]
- 8. Ebner J, Pan X, Yue Y, Sideromenos S, Marksteiner J, Koenig X, Hilber K, Duan D. Microdystrophin therapy rescues impaired Na currents in cardiac Purkinje fibers from dystrophin-deficient mdx mice. Circ Arrhythm Electrophysiol 15: e011161, 2022. doi: 10.1161/CIRCEP.122.011161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, , et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 385: 1451–1461, 2021. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 10. Chen X, Hocher CF, Shen L, Krämer BK, Hocher B. Reno- and cardioprotective molecular mechanisms of SGLT2 inhibitors beyond glycemic control: from bedside to bench. Am J Physiol Cell Physiol 325: C661–C681, 2023. doi: 10.1152/ajpcell.00177.2023. [DOI] [PubMed] [Google Scholar]
- 11. Philippaert K, Kalyaanamoorthy S, Fatehi M, Long W, Soni S, Byrne NJ, Barr A, Singh J, Wong J, Palechuk T, Schneider C, Darwesh AM, Maayah ZH, Seubert JM, Barakat K, Dyck JRB, Light PE. Cardiac late sodium channel current is a molecular target for the sodium/glucose cotransporter 2 inhibitor empagliflozin. Circulation 143: 2188–2204, 2021. doi: 10.1161/CIRCULATIONAHA.121.053350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hegyi B, Mira Hernandez J, Shen EY, Habibi NR, Bossuyt J, Bers DM. Empagliflozin reverses late Na+ current enhancement and cardiomyocyte proarrhythmia in a translational murine model of heart failure with preserved ejection fraction. Circulation 145: 1029–1031, 2022. doi: 10.1161/CIRCULATIONAHA.121.057237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mustroph J, Baier MJ, Pabel S, Stehle T, Trum M, Provaznik Z, Mohler PJ, Musa H, Hund TJ, Sossalla S, Maier LS, Wagner S. Empagliflozin inhibits cardiac late sodium current by Ca/calmodulin-dependent kinase II. Circulation 146: 1259–1261, 2022. [Erratum in Circulation 146: e325, 2022]. doi: 10.1161/CIRCULATIONAHA.122.057364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dago M, Crespo-García T, Cámara-Checa A, Rapún J, Rubio-Alarcón M, Marín M, Tamargo J, Caballero R, Delpón E. Empagliflozin and dapagliflozin increase Na+ and inward rectifier K+ current densities in human cardiomyocytes derived from induced pluripotent stem cells (hiPSC-CMs). Cells 11: 3707, 2022. doi: 10.3390/cells11233707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haffner V, Nourian Z, Boerman EM, Lambert MD, Hanft LM, Krenz M, Baines CP, Duan D, McDonald KS, Domeier TL. Calcium handling dysfunction and cardiac damage following acute ventricular preload challenge in the dystrophin-deficient mouse heart. Am J Physiol Heart Circ Physiol 325: H1168–H1177, 2023. doi: 10.1152/ajpheart.00265.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pennig J, Scherrer P, Gissler MC, Anto-Michel N, Hoppe N, Füner L, Härdtner C, Stachon P, Wolf D, Hilgendorf I, Mullick A, Bode C, Zirlik A, Goldberg IJ, Willecke F. Glucose lowering by SGLT2-inhibitor empagliflozin accelerates atherosclerosis regression in hyperglycemic STZ-diabetic mice. Sci Rep 9: 17937, 2019. doi: 10.1038/s41598-019-54224-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Østergaard MV, Secher T, Christensen M, Salinas CG, Roostalu U, Skytte JL, Rune I, Hansen HH, Jelsing J, Vrang N, Fink LN. Therapeutic effects of lisinopril and empagliflozin in a mouse model of hypertension-accelerated diabetic kidney disease. Am J Physiol Renal Physiol 321: F149–F161, 2021. doi: 10.1152/ajprenal.00154.2021. [DOI] [PubMed] [Google Scholar]
- 18. Gallo LA, Ward MS, Fotheringham AK, Zhuang A, Borg DJ, Flemming NB, Harvie BM, Kinneally TL, Yeh SM, McCarthy DA, Koepsell H, Vallon V, Pollock C, Panchapakesan U, Forbes JM. Once daily administration of the SGLT2 inhibitor, empagliflozin, attenuates markers of renal fibrosis without improving albuminuria in diabetic db/db mice. Sci Rep 6: 26428, 2016. [Erratum in Sci Rep 6: 28124, 2016]. doi: 10.1038/srep26428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tauber P, Sinha F, Berger RS, Gronwald W, Dettmer K, Kuhn M, Trum M, Maier LS, Wagner S, Schweda F. Empagliflozin reduces renal hyperfiltration in response to uninephrectomy, but is not nephroprotective in UNx/DOCA/salt mouse models. Front Pharmacol 12: 761855, 2021. doi: 10.3389/fphar.2021.761855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sikkel MB, Francis DP, Howard J, Gordon F, Rowlands C, Peters NS, Lyon AR, Harding SE, MacLeod KT. Hierarchical statistical techniques are necessary to draw reliable conclusions from analysis of isolated cardiomyocyte studies. Cardiovasc Res 113: 1743–1752, 2017. doi: 10.1093/cvr/cvx151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iborra-Egea O, Santiago-Vacas E, Yurista SR, Lupón J, Packer M, Heymans S, Zannad F, Butler J, Pascual-Figal D, Lax A, Núñez J, de Boer RA, Bayés-Genís A. Unraveling the molecular mechanism of action of empagliflozin in heart failure with reduced ejection fraction with or without diabetes. JACC Basic Transl Sci 4: 831–840, 2019. doi: 10.1016/j.jacbts.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bayes-Genis A, Iborra-Egea O, Spitaleri G, Domingo M, Revuelta-López E, Codina P, Cediel G, Santiago-Vacas E, Cserkóová A, Pascual-Figal D, Núñez J, Lupón J. Decoding empagliflozin’s molecular mechanism of action in heart failure with preserved ejection fraction using artificial intelligence. Sci Rep 11: 12025, 2021. doi: 10.1038/s41598-021-91546-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dyck JRB, Sossalla S, Hamdani N, Coronel R, Weber NC, Light PE, Zuurbier CJ. Cardiac mechanisms of the beneficial effects of SGLT2 inhibitors in heart failure: evidence for potential off-target effects. J Mol Cell Cardiol 167: 17–31, 2022. doi: 10.1016/j.yjmcc.2022.03.005. [DOI] [PubMed] [Google Scholar]
- 24. Park SH, Belcastro E, Hasan H, Matsushita K, Marchandot B, Abbas M, Toti F, Auger C, Jesel L, Ohlmann P, Morel O, Schini-Kerth VB. Angiotensin II-induced upregulation of SGLT1 and 2 contributes to human microparticle-stimulated endothelial senescence and dysfunction: protective effect of gliflozins. Cardiovasc Diabetol 20: 65, 2021. doi: 10.1186/s12933-021-01252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakao M, Shimizu I, Katsuumi G, Yoshida Y, Suda M, Hayashi Y, Ikegami R, Hsiao YT, Okuda S, Soga T, Minamino T. Empagliflozin maintains capillarization and improves cardiac function in a murine model of left ventricular pressure overload. Sci Rep 11: 18384, 2021. doi: 10.1038/s41598-021-97787-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tao W, Yang X, Zhang Q, Bi S, Yao Z. Optimal treatment for post-MI heart failure in rats: dapagliflozin first, adding sacubitril-valsartan 2 weeks later. Front Cardiovasc Med 10: 1181473, 2023. doi: 10.3389/fcvm.2023.1181473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hegyi B, Bers DM. New cardiac targets for empagliflozin: O-GlcNAcylation, CaMKII, and calcium handling. Am J Physiol Heart Circ Physiol 324: H338–H340, 2023. doi: 10.1152/ajpheart.00003.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yoshii A, Nagoshi T, Kashiwagi Y, Kimura H, Tanaka Y, Oi Y, Ito K, Yoshino T, Tanaka TD, Yoshimura M. Cardiac ischemia-reperfusion injury under insulin-resistant conditions: SGLT1 but not SGLT2 plays a compensatory protective role in diet-induced obesity. Cardiovasc Diabetol 18: 85, 2019. doi: 10.1186/s12933-019-0889-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaplan A, Abidi E, El-Yazbi A, Eid A, Booz GW, Zouein FA. Direct cardiovascular impact of SGLT2 inhibitors: mechanisms and effects. Heart Fail Rev 23: 419–437, 2018. doi: 10.1007/s10741-017-9665-9. [DOI] [PubMed] [Google Scholar]
- 30. Mustroph J, Wagemann O, Lücht CM, Trum M, Hammer KP, Sag CM, Lebek S, Tarnowski D, Reinders J, Perbellini F, Terracciano C, Schmid C, Schopka S, Hilker M, Zausig Y, Pabel S, Sossalla ST, Schweda F, Maier LS, Wagner S. Empagliflozin reduces Ca/calmodulin-dependent kinase II activity in isolated ventricular cardiomyocytes. ESC Heart Fail 5: 642–648, 2018. doi: 10.1002/ehf2.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cappetta D, De Angelis A, Ciuffreda LP, Coppini R, Cozzolino A, Miccichè A, Dell'Aversana C, D’Amario D, Cianflone E, Scavone C, Santini L, Palandri C, Naviglio S, Crea F, Rota M, Altucci L, Rossi F, Capuano A, Urbanek K, Berrino L. Amelioration of diastolic dysfunction by dapagliflozin in a non-diabetic model involves coronary endothelium. Pharmacol Res 157: 104781, 2020. doi: 10.1016/j.phrs.2020.104781. [DOI] [PubMed] [Google Scholar]
- 32. Kadosaka T, Watanabe M, Natsui H, Koizumi T, Nakao M, Koya T, Hagiwara H, Kamada R, Temma T, Karube F, Fujiyama F, Anzai T. Empagliflozin attenuates arrhythmogenesis in diabetic cardiomyopathy by normalizing intracellular Ca2+ handling in ventricular cardiomyocytes. Am J Physiol Heart Cir0c Physiol 324: H341–H354, 2023. doi: 10.1152/ajpheart.00391.2022. [DOI] [PubMed] [Google Scholar]
- 33. Offord J, Catterall WA. Electrical activity, cAMP, and cytosolic calcium regulate mRNA encoding sodium channel alpha subunits in rat muscle cells. Neuron 2: 1447–1452, 1989. doi: 10.1016/0896-6273(89)90190-6. [DOI] [PubMed] [Google Scholar]
- 34. Duff HJ, Offord J, West J, Catterall WA. Class I and IV antiarrhythmic drugs and cytosolic calcium regulate mRNA encoding the sodium channel alpha subunit in rat cardiac muscle. Mol Pharmacol 42: 570–574, 1992. [PubMed] [Google Scholar]
- 35. Takla M, Huang CL, Jeevaratnam K. The cardiac CaMKII-Nav1.5 relationship: from physiology to pathology. J Mol Cell Cardiol 139: 190–200, 2020. doi: 10.1016/j.yjmcc.2019.12.014. [DOI] [PubMed] [Google Scholar]
- 36. Martin CA, Zhang Y, Grace AA, Huang CL. In vivo studies of Scn5a+/− mice modeling Brugada syndrome demonstrate both conduction and repolarization abnormalities. J Electrocardiol 43: 433–439, 2010. doi: 10.1016/j.jelectrocard.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martin CA, Guzadhur L, Grace AA, Lei M, Huang CL. Mapping of reentrant spontaneous polymorphic ventricular tachycardia in a Scn5a+/− mouse model. Am J Physiol Heart Circ Physiol 300: H1853–H1862, 2011. doi: 10.1152/ajpheart.00034.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.