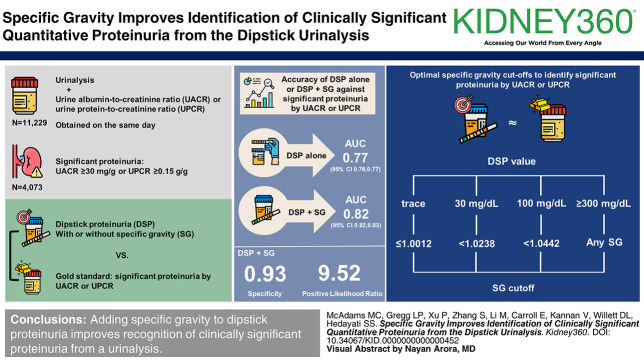
Visual Abstract
Keywords: CKD, proteinuria
Abstract
Key Points
Urine albumin-to-creatinine ratio and urine protein-to-creatinine ratio are frequently obtained and represent possible tools for screening for proteinuria and thus early CKD.
Adding specific gravity to dipstick proteinuria improves the ability to screen patients with clinically significant proteinuria and can be used to identify patients with early CKD.
Background
CKD is often underdiagnosed during early stages when GFR is preserved because of underutilization of testing for quantitative urine albumin-to-creatinine ratio (UACR) or urine protein-to-creatinine ratio (UPCR). Semiquantitative dipstick proteinuria (DSP) on urinalysis is widely obtained but not accurate for identifying clinically significant proteinuria.
Methods
We identified all patients with a urinalysis and UACR or UPCR obtained on the same day at a tertiary referral center. The accuracy of DSP alone or in combination with specific gravity (SG) against a gold-standard UACR ≥30 mg/g or UPCR ≥0.15 g/g, characterizing clinically significant proteinuria, was evaluated using logistic regression. Models were internally validated using ten-fold cross-validation. The SG for each DSP above which significant proteinuria is unlikely was determined.
Results
Of 11,229 patients, clinically significant proteinuria was present in 4073 (36%). The area under the receiver-operating characteristic curve (95% confidence interval) was 0.77 (0.76 to 0.77) using DSP alone and 0.82 (0.82 to 0.83) in combination with SG (P < 0.001), yielding a specificity of 0.93 (SEM=0.02) and positive likelihood ratio of 9.52 (SEM=0.85). The optimal SG cutoffs to identify significant proteinuria were ≤1.0012, 1.0238, and 1.0442 for DSP of trace, 30, and 100 mg/dl, respectively. At any SG, a DSP ≥300 mg/dl was extremely likely to represent significant proteinuria.
Conclusions
Adding SG to DSP improves recognition of clinically significant proteinuria and can be easily used to identify patients with early stage CKD who may not have otherwise received a quantified proteinuria measurement for both clinical and research purposes.
Introduction
CKD remains frequently underdiagnosed in early stages when GFR is preserved because of underutilization of quantitative testing for clinically significant proteinuria in general practice.1,2 However, it is at the early stages where implementation of interventions that would have the most impactful effects on outcomes in patients with CKD become imperative. A urine albumin-to-creatinine ratio (UACR) and urine protein-to-creatinine ratio (UPCR) from a random urine sample provide accurate quantitative results and are less cumbersome to obtain than 24-hour urine collection.3 Clinically significant proteinuria is defined as a UACR ≥30 m/g or UPCR ≥0.15 g/g.1 However, there is currently no guideline recommending UACR or UPCR to screen individuals without known CKD or diabetes. Consequently, proteinuria, particularly among patients with an eGFR ≥60 ml/min per 1.73 m2, may remain undiagnosed and untreated.
Dipstick urinalysis, on the other hand, is widely available and frequently obtained. Among several colorimetric assays reported on a dipstick urinalysis is a semiqualitative measure of proteinuria. Unlike quantitative UACR and UPCR results, interpretation of dipstick proteinuria (DSP) results depends on the concentration of the urine sample, which can be approximated by the concomitantly reported specific gravity (SG). In other words, DSP may be falsely negative in dilute urine or falsely elevated in very concentrated urine. Previous studies that evaluated the relationship of DSP with UACR or UPCR did not consider the contribution of SG.4,5 Combining DSP with urine SG has shown promise as a screening method for proteinuria in pregnant patients or to identify severe proteinuria,6,7 but has not been studied for current guideline-based thresholds of proteinuria to identify patients with proteinuria.
Because dipstick urinalysis is widely clinically performed, it may be useful for creating a pragmatic tool within the electronic health record (EHR) to identify patients who may benefit from further evaluation and intervention with a quantitative proteinuria measurement. Accurate identification of patients with early stage CKD before eGFR decline could enhance early implementation of interventions, such as angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and sodium-glucose cotransporter 2 inhibitors, to slow the progression of kidney disease and improve cardiovascular outcomes.8–11 This would also allow more patients with CKD stages 1 and 2 to be easily identified for research using real-world data, which is important because these patients are excluded from most studies that define CKD by eGFR cutoffs alone. Therefore, we aimed to (1) assess the accuracy of the DSP, SG, and the combination of the two for identifying patients with clinically significant quantified proteinuria and (2) identify the ideal SG cutoff for each DSP value above which clinically significant proteinuria is unlikely. We hypothesized that adding SG to DSP would more accurately predict clinically significant proteinuria than DSP alone.
Methods
Patient Population
Using a pragmatic diagnostic test study approach, we used the established National Kidney Disease Education Program (NKDEP) recommendations12 as an e-phenotype tool to identify patients with CKD (eGFR <60 ml/min per 1.73 m2 or UACR ≥30 mg/g or UPCR ≥0.15 g/g with two values >90 days apart) using data widely available in the EHR (EPIC systems, Verona, WI) at the University of Texas Southwestern Medical Center Clements University Hospital in Dallas, Texas (Supplemental Table 1). Patients 18 years and older were included if they had a UACR or UPCR obtained within the same 24-hour period as a dipstick urinalysis that included DSP and SG between January 1, 2019, and December 31, 2021. For individuals with multiple dates meeting inclusion criteria during the study period, only the most recent DSP and UACR/UPCR pair was used in the analysis. Patient information was deidentified. The study was approved by the University of Texas Southwestern Institutional Review Board as exempt from human subjects' research, including a waiver of informed consent.
Participant Characteristics
Demographics were obtained from the EHR as they were listed on the date the urinary data were obtained. Individuals were considered as having a comorbidity if the diagnosis was listed in the EHR as an active problem and/or there was an encounter diagnosis within the past 3 years. eGFR was calculated from the serum creatinine value in the EHR on the date of collection of the DSP and UPCR/UACR pair using the 2021 non–race-based CKD Epidemiology Collaboration Creatinine Equation.13 To maximize external validity, there were no exclusion criteria related to eGFR, comorbidities, inpatient or outpatient status, or urologic surgical history.
Dipstick Urinalysis Measurements
The predictor variables were DSP and SG reported on a dipstick urinalysis, identified using Logical Observation Identifier Names and Codes from the NKDEP ePhenotype (Supplemental Table 1). DSP was reported as negative, trace, 30, 100, or ≥300 mg/dl. Negative and trace were grouped together as a single category for this analysis. Some values for DSP performed at an outside laboratory were reported as +, ++, +++, or ++++. These were excluded because only 4% of the results were reported using these values.
SG was considered as a continuous variable, reported as a unit-less value from 1.005 to 1.030. Values reported as <1.005 were converted to 1.005 and values >1.030 were converted to 1.030 for the analysis. Some values were reported as less than or greater than a value other than 1.005 or 1.030; for example, if the SG was reported as >1.050, then 1.050 was used, or if the SG was reported as <1.010, then 1.010 was used.
Outcome Variable
Clinically significant proteinuria was defined as UACR ≥30 mg/g or UPCR ≥0.15 g/g. UACR and UPCR tests were identified from the EHR using Logical Observation Identifier Names and Codes from the NKDEP ePhenotype (Supplemental Table 1).12 Individuals who met at least one of these thresholds were coded as meeting criteria for clinically significant proteinuria. Only UACR and UPCR values from random urine samples were included. Some UACR or UPCR results were reported as below a lower threshold (e.g., <0.1 mg/g). These were considered negative for clinically significant proteinuria.
Statistical Analysis
Continuous variables were presented as mean±SD or median (25th–75th percentile), and categorical variables were described using count and percentages. To compare across DSP categories, the Cochran–Armitage trend test was used for binary categorical variables, generalized Pearson chi-square test for nominal categorical variables with more than two levels, and linear-by-linear association test for ordinal categorical variables with more than two levels. We applied the Jonckheere–Terpstra test for continuous variables. Pairwise comparisons were performed when the overall test was significant, and the adjustment of P values for multiple comparisons was based on the Holm method.
We used a diagnostic test study design and logistic regression to evaluate the accuracy of urinalysis DSP alone or in combination with urinalysis SG against a gold-standard test of UACR ≥30 mg/g or UPCR ≥0.15 g/g. Receiver-operating characteristic curves were generated, and the areas under the curve (AUC) were compared using DeLong tests. At each level of DSP, the relationship between SG and probability of clinically significant proteinuria was plotted and the optimal cutoffs for SG were obtain on the basis of the J-statistic (Youden index).14 Odds ratios (ORs) with 95% confidence intervals (CIs) were reported using negative/trace as the reference group. Models were internally validated using ten-fold cross-validation.
Models were tested for multicollinearity using variance inflation factors, with a variance inflation factor greater than ten suggesting evidence of multicollinearity.15 Model evaluation indices contained sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR), and negative likelihood ratio (NLR), shown as an average (±SEM) across ten-fold stratified cross-validation. We used the Youden index from the receiver-operating characteristic curve analysis to identify the optimal predicted probability cutoff for proteinuria. We derived mathematic equations to calculate the predicted probability of proteinuria. The model discrimination was quantified using AUC, and the model calibration was accessed with calibration curves.16 Model performance in subgroups were also derived, and bootstrap percentile-based CIs were obtained from 2000 bootstrap samples. P values obtained from bootstrapping were adjusted with the Holm method to adjust familywise error rate.
Statistical analyses were performed with R software, version R-4.1.0 (R Foundation for Statistical Computing, Vienna, Austria). A two-sided P value < 0.05 was considered statistically different.
Results
Participant Characteristics
A total of 11,229 participants met inclusion criteria. The mean (SD) age was 58.3 (17.0) years, and 46% were male (Table 1). Of the 9756 individuals with available race information, 6017 (62%) were White and 2502 (26%) were Black. There were 3090 (28%) with diabetes mellitus and 5756 (51%) with hypertension.
Table 1.
Baseline characteristics overall and by dipstick proteinuria value
| Characteristics, N (%) | Overall N=11,229 |
Negative/Trace n=7956 |
30 mg/dl n=1700 |
100 mg/dl n=1219 |
≥300 mg/dl n=354 |
P Value |
|---|---|---|---|---|---|---|
| Male sex, n/total n (%) | 5165/11,227 (46.0) | 3549/7954 (44.6)2c | 807 (47.5) | 629 (51.6) | 180 (50.8) | <0.001 |
| Age, yr, mean±SD | 58.3±17.0 | 58.0±16.71a,2c | 58.9±17.8 | 59.9±17.46a | 57.4±17.4 | <0.001 |
| Race, n/total n (%) | <0.001a | |||||
| White | 6017/9756 (61.7) | 4346/6804 (63.9) | 896/1527 (58.7) | 623/1103 (56.5) | 152/322 (47.2) | |
| Black | 2502/9756 (25.6) | 1500/6804 (22.0) | 492/1527 (32.2) | 382/1103 (34.6) | 128/322 (39.8) | |
| Asian | 844/9756 (8.7) | 700/6804 (10.3) | 79/1527 (5.2) | 49/1103 (4.4) | 16/322 (5.0) | |
| American Indian or Alaska Native | 49/9756 (0.5) | 35/6804 (0.5) | 8/1527 (0.5) | 5/1103 (0.5) | 1/322 (0.3) | |
| Native Hawaiian or Other Pacific Islander | 16/9756 (0.2) | 11/6804 (0.2) | 3/1527 (0.2) | 2/1103 (0.2) | 0/322 (0.0) | |
| Other | 328/9756 (3.4) | 212/6804 (3.1) | 49/1527 (3.2) | 42/1103 (3.8) | 25/322 (7.8) | |
| Diabetes mellitus, n (%) | 3090 (27.5) | 1828 (23.0)1c,2c,3c | 591 (34.8)4b,5c | 494 (40.5)6b | 177 (50.0) | <0.001 |
| Hypertension, n (%) | 5756 (51.3) | 3640 (45.8)1c,2c,3c | 1046 (61.5)4b,5b | 818 (67.1) | 252 (71.2) | <0.001 |
| CAD, n/total n (%) | 1331/6203 (21.5) | 803/3932 (20.4) | 262/1137 (23.0) | 212/867 (24.5) | 54/267 (20.2) | 0.04 |
| ACEi/ARB, n/total n (%) | 3370/6203 (54.3) | 2124/3932 (54.0) | 624/1137 (54.9) | 493/867 (56.9) | 129/267 (48.3) | 0.9 |
| SG, median (IQR) | 1.016 (1.011–1.021) | 1.015 (1.011–1.020)1c,2c,3b | 1.019 (1.014–1.025)4c,5c | 1.016 (1.012–1.021) | 1.016 (1.013, 1.020) | <0.001 |
| eGFR, mean±SD, ml/min per 1.73 m2 | 71.81±31.48 | 77.49±28.061c,2c,3c | 67.24±33.504c,5c | 51.51±33.576c | 39.06±32.46 | <0.001 |
| eGFR ≥60 ml/min per 1.73 m2, n/total n (%) | 6122/9722 (63.0) | 4896/6868 (71.3)1c,2c,3c | 808/1463 (55.2)4c,5c | 352/1065 (33.1)6c | 66/326 (20.2) | <0.001 |
| eGFR cutoffs, ml/min per 1.73 m 2 , n/total n (%) | <0.001b | |||||
| ≥90 | 3161/9722 (32.5) | 2518/6868 (36.7) | 439/1463 (30.0) | 168/1065 (15.8) | 36/326 (11.0) | |
| 60–89 | 2961/9722 (30.5) | 2378/6868 (34.6) | 369/1463 (25.2) | 184/1065 (17.3) | 30/326 (9.2) | |
| 45–59 | 1389/9722 (14.3) | 985/6868 (14.3) | 202/1463 (13.8) | 170/1065 (16.0) | 32/326 (9.8) | |
| 30–44 | 1131/9722 (11.6) | 642/6868 (9.3) | 219/1463 (15.0) | 209/1065 (19.6) | 61/326 (18.7) | |
| 15–29 | 746/9722 (7.7) | 299/6868 (4.4) | 159/1463 (10.9) | 200/1065 (18.8) | 88/326 (27.0) | |
| <15 | 334/9722 (3.4) | 46/6868 (0.7) | 75/1463 (5.1) | 134/1065 (12.6) | 79/326 (24.2) | |
To compare across dipstick proteinuria categories, we used the Cochran–Armitage trend test for binary categorical variables, generalized Pearson chi-square test for the nominal categorical variable with more than two levels and linear-by-linear association test for ordinal categorical variable with more than two levels. We applied the Jonckheere–Terpstra test for continuous variables. Pairwise comparisons among negative or trace, 30, 100, and ≥300 mg/dl were performed when the overall test was significant, and the adjustment to P values was based on the Holm method. Pairwise for race value and GFR cutoffs are for overall differences. ACEi/ARB, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers; CAD, coronary artery disease; IQR, interquartile range; SG, specific gravity; UACR, urine albumin-to-creatinine ratio; UPCR, urine protein-to-creatinine ratio.
Pairwise comparison between negative/trace and 30 mg/dl: 1aP < 0.05, 1bP < 0.01, 1cP < 0.001.
Pairwise comparison between negative/trace and 100 mg/dl: 2aP < 0.05, 2bP < 0.01, 2cP < 0.001.
Pairwise comparison between negative/trace and ≥300 mg/dl: 3aP < 0.05, 3bP < 0.01, 3cP < 0.001.
Pairwise comparison between 30 and 100 mg/dl: 4aP < 0.05, 4bP < 0.01, 4cP < 0.001.
Pairwise comparison between 30 and ≥300 mg/dl: 5aP < 0.05, 5bP < 0.01, 5cP < 0.001.
Pairwise comparison between 100 and ≥300 mg/dl: 6aP < 0.05, 6bP < 0.01, 6cP < 0.001.
Pairwise comparison for overall race variable: 1c, 2c, 3c, 5c, 6a.
Pairwise comparison for overall eGFR cutoffs: 1c, 2c, 3c, 4c, 5c, 6c
On dipstick urinalysis, 7956 patients (71%) had a DSP value of negative or trace, 1700 (15%) 30 mg/dl, 1219 (11%) 100 mg/dl, and 354 (3%) ≥300 mg/dl. The median SG of all samples was 1.016 (interquartile range, 1.011–1.021). As DSP increased, the proportion of individuals with diabetes mellitus increased (Table 1). All pairwise comparisons were significant. The proportion with hypertension also increased as DSP increased (P < 0.05 for each pairwise comparison, except for DSP ≥300 mg/dl versus 100 mg/dl, P = 0.17). Mean (SD) eGFR for the entire cohort was 71.8 (31.5) ml/min per 1.73 m2, with 6122 patients (63%) having an eGFR ≥60 ml/min per 1.73 m2. As DSP increased, the proportion of patients with eGFR ≥60 ml/min per 1.73 m2 decreased (all pairwise P < 0.05).
Of the entire cohort, 4073 (36%) met criteria for clinically significant proteinuria by UACR or UPCR (Table 2). Of the 5701 participants with a UACR, 1974 (34.6%) had a value ≥30 mg/g. Of the 6925 participants with a UPCR, 2677 (38.7%) had a value ≥0.15 g/g. There were 4073 patients (36.3%) in the full cohort who met the criteria for clinically significant proteinuria, including 1595 (20%) of the negative/trace DSP group, 1010 (59%) of those with DSP 30 mg/dl, 1119 (92%) with DSP 100 mg/dl, and 349 (99%) with DSP ≥300 mg/dl. All pairwise comparisons were significant (P < 0.05).
Table 2.
Urine protein-to-creatinine ratio and urine albumin-to-creatinine ratio cutoffs by dipstick proteinuria category
| UPCR/UACR Value | All Patients N=11,229 n/Total N (%) |
DSP=Negative/Trace n=7956 n/Total N (%) |
DSP=30 mg/dl n=1700 n/Total N (%) |
DSP=100 mg/dl n=1219 n/Total N (%) |
DSP=≥300 mg/dl n=354 n/Total N (%) |
Omnibus P Value |
|---|---|---|---|---|---|---|
| UPCR ≥0.15 g/g | 2677/6925 (38.7) | 884/4573 (19.3)1c,2c,3c | 671/1146 (58.6)4c,5c | 846/926 (91.4)6c | 276/280 (98.6) | <0.001 |
| UPCR<0.15 g/g | 4248/6925 (61.3) | 3689/4573 (80.7) | 475/1146 (41.4) | 80/926 (8.6) | 4/280 (1.4) | |
| UACR ≥30 mg/g | 1974/5701 (34.6) | 863/4222 (20.4)1c,2c,3c | 504/839 (60.1)4c,5c | 489/521 (93.9)6a | 118/119 (99.2) | <0.001 |
| UACR<30 mg/g | 3727/5701 (65.4) | 3359/4222 (79.6) | 335/839 (39.9) | 32/521 (6.1) | 1/119 (0.8) | |
| UPCR ≥0.15 g/g or UACR ≥30 mg/g | 4073/11,229 (36.3) | 1595/7256 (20.0)1c,2c,3c | 1010/1700 (59.4)4c,5c | 1119/1219 (91.8)6c | 349/354 (98.6) | <0.001 |
| Neither UPCR ≥0.15 g/g or UACR ≥30 mg/g | 7156/11,229 (63.7) | 6361/7256 (80.0) | 690/1700 (40.6) | 100/1219 (8.2) | 5/354 (1.4) |
To compare across dipstick proteinuria categories, we used the Cochran–Armitage trend test for binary categorical variables, generalized Pearson chi-square test for the nominal categorical variable with more than two levels, and linear-by-linear association test for ordinal categorical variable with more than two levels. We applied the Jonckheere–Terpstra test for continuous variables. Pairwise comparisons among negative or trace, 30, 100, and ≥300 mg/dl were performed for gold-standard proteinuria cutoffs when the overall test was significant, and the adjustment to P values was based on the Holm method. DSP, dipstick proteinuria; UACR, urine albumin-to-creatinine ratio; UPCR, urine protein-to-creatinine ratio.
Pairwise comparison between negative/trace and 30 mg/dl: 1aP < 0.05, 1bP < 0.01, 1cP < 0.001.
Pairwise comparison between negative/trace and 100 mg/dl: 2aP < 0.05, 2bP < 0.01, 2cP < 0.001.
Pairwise comparison between negative/trace and ≥300 mg/dl: 3aP < 0.05, 3bP < 0.01, 3cP < 0.001.
Pairwise comparison between 30 and 100 mg/dl: 4aP < 0.05, 4bP < 0.01, 4cP < 0.001.
Pairwise comparison between 30 and ≥300 mg/dl: 5aP < 0.05, 5bP < 0.01, 5cP < 0.001.
Pairwise comparison between 100 and ≥300 mg/dl: 6aP < 0.05, 6bP < 0.01, 6cP < 0.001.
Models to Predict Clinically Significant Proteinuria
In univariable models, a DSP value of 30 mg/dl compared with negative/trace was associated with clinically significant proteinuria (OR, 5.8; 95% CI, 5.2 to 6.5; P < 0.001). When SG was added to the model, the OR increased to 9.9 (95% CI, 8.7 to 11.3, P < 0.001). A DSP value of 100 mg/dl had an OR of 44.6 (95% CI, 36.1 to 55.2) in univariable analysis and 77.7 (95% CI, 61.7 to 97.8, P < 0.001) when adjusting for SG. DSP ≥300 mg/dl had a univariable OR of 278.4 (95% CI, 115.0 to 673.8) in the unadjusted model and 495.9 (95% CI, 202.0 to 1217.3, P < 0.001) in the adjusted model (Supplemental Figure 1).
The AUC in ten-fold cross-validation models to predict clinically significant proteinuria was 0.60 (95% CI, 0.56 to 0.61) for SG alone, 0.77 (95% CI, 0.76 to 0.77) for DSP alone, and 0.82 (0.82 to 0.83) for the combination of DSP and SG (Figure 1A). The model with both DSP and SG had a significantly higher AUC compared with the models using SG only (P < 0.001) and DSP only (P < 0.001). Calibration curves show the best calibration for the model including both SG and DSP to predict clinically significant proteinuria (Figure 1B). Metrics for models to predict clinically significant proteinuria are presented in Table 3. The model that combined the DSP value with SG had a specificity of 0.93 (SEM=0.015) and PLR of 9.52 (SEM=0.847) for predicting clinically significant proteinuria.
Figure 1.
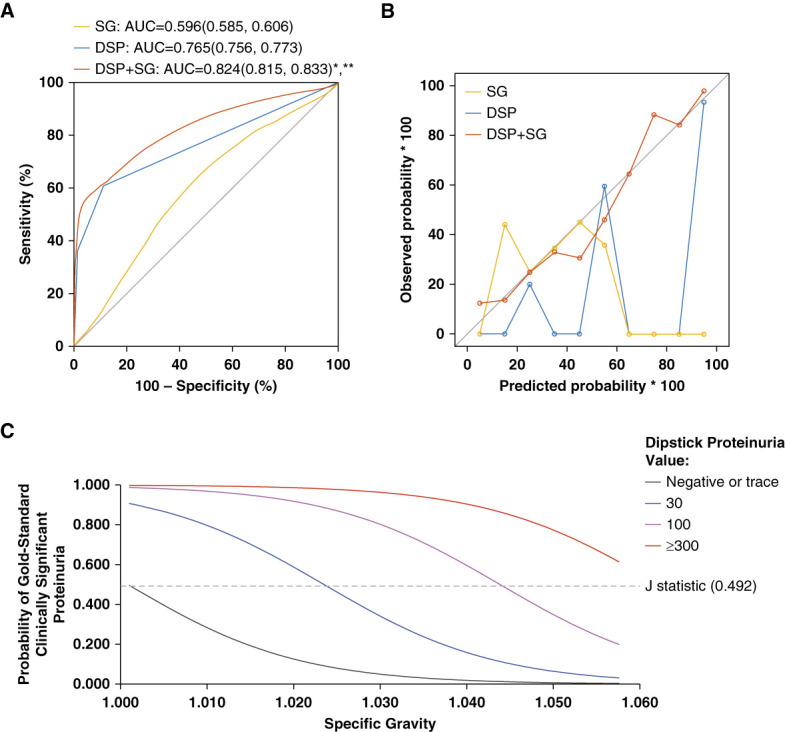
Performance of specific gravity plus dipstick proteinuria over dipstick proteinuria alone for the prediction of clinically significant proteinuria. (A) Receiver operator characteristic curves and (B) calibration curves of SG, DSP, and SG and DSP for the outcome of clinically significant proteinuria. Calibration curves indicate the predicted probability of clinically significant proteinuria, treated as a continuous variable, relative to the observed probability. *DSP and SG compared with SG P value < 0.001. ** DSP and SG compared with DSP alone P value < 0.001. (C) SG cutoffs to predict gold standard proteinuria for each level of DSP results calculated on the basis of the J-statistic. AUC, areas under the curve; DSP, dipstick proteinuria; SG, specific gravity.
Table 3.
Ten-fold cross-validation metrics for clinically significant proteinuria models
| Metric Mean±SEM |
DSP | SG | DSP+SG |
|---|---|---|---|
| AUC | 0.765±0.003 | 0.596±0.006 | 0.824±0.005 |
| Sensitivity | 0.608±0.006 | 0.619±0.028 | 0.597±0.017 |
| Specificity | 0.889±0.003 | 0.558±0.025 | 0.926±0.015 |
| PPV | 0.757±0.005 | 0.444±0.004 | 0.832±0.019 |
| NPV | 0.800±0.003 | 0.722±0.006 | 0.802±0.005 |
| PLR | 5.513±0.161 | 1.406±0.023 | 9.52±0.847 |
| NLR | 0.441±0.007 | 0.677±0.022 | 0.434±0.013 |
AUC, areas under the curve; DSP, dipstick proteinuria; NLR, negative likelihood ratio; NPV, negative predictive value; PLR, positive likelihood ratio; PPV, positive predictive value; SG, specific gravity.
We developed an equation to determine the probability of clinically significant proteinuria using both the DSP and SG. A value of 1 is entered into the equation to specify the patient's DSP value, and 0 is entered for all other DSP values:
Optimal Cutoffs for SG
The SG cutoff needed to predict clinically significant proteinuria on the basis of the J statistic for each DSP value is illustrated in Figure 1C. The optimal SG for each DSP level is represented as the point where the curve intersects the J-index line. For a DSP of 30 mg/dl, a SG of ≤1.0238 is needed. A SG ≤1.0442 is optimal for samples with DSP 100 mg/dl. The optimal SG cutoff value for DSP ≥300 mg/dl is 1.060. Because this value is higher than the upper reported limit for SG, at any SG, a DSP value ≥300 mg/dl is extremely likely to represent clinically significant proteinuria.
Validation in Subgroups
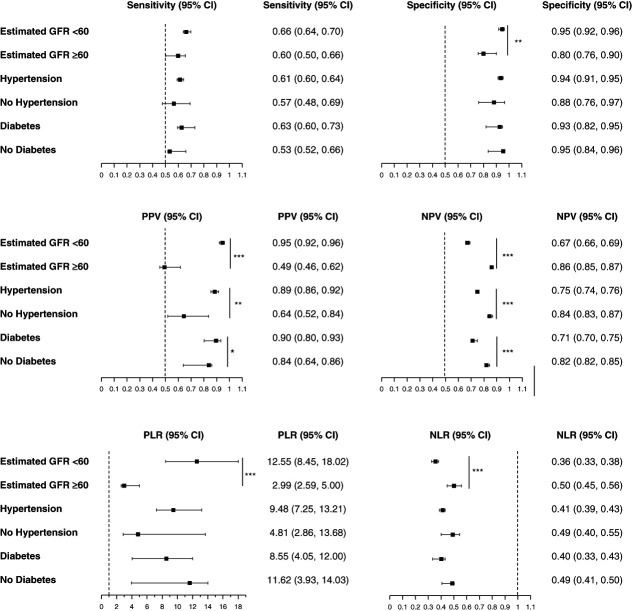
Performance metrics of the model to predict clinically significant proteinuria using both DSP and SG were assessed in various subgroups (Figure 2). There were no significant differences in the sensitivity, specificity, PLR, and NLR of the model in patients with or without diabetes or in patients with or without hypertension. The PPV was higher in patients with diabetes (0.90, 95% CI, 0.80 to 0.92) than in those with no diabetes (0.84, 95% CI, 0.64 to 0.86, P = 0.03). The NPV was higher in those without diabetes (0.82, 95% CI, 0.82 to 0.85), than in those with diabetes (0.71, 95% CI, 0.70 to 0.75, P < 0.001). Similarly, the PPV was higher in those with hypertension (0.89, 95% CI, 0.86 to 0.92) compared with those with no hypertension (0.64, 95% CI, 0.52 to 0.84, P = 0.002), and the NPV was higher in those without hypertension (0.84, 95% CI, 0.83 to 0.0.87, P < 0.001).
Figure 2.
Model performance metrics for detecting proteinuria from DSP and SG in cohort subpopulations. Metrics include sensitivity, specificity, PPV, NPV, PLR, and NLR with corresponding 95% CI. Subpopulations include eGFR <60 ml/min per 1.73 m2, eGFR ≥60 ml/min per 1.73 m2, hypertension, no hypertension, diabetes, and no diabetes. ***P < 0.001, **P < 0.01, *P < 0.05. CI, confidence interval; NLR, negative likelihood ratio; NPV, negative predictive value; PLR, positive likelihood ratio; PPV, positive predictive value.
The sensitivity of the model did not differ in those with an eGFR ≥60 ml/min per 1.73 m2 and those with an eGFR <60 ml/min per 1.73 m2. However, the specificity, PPV, and PLR were higher in those with eGFR <60 ml/min per 1.73 m2, and the NPV and NLR were higher in those with eGFR ≥60 ml/min per 1.73 m2. Performance metrics by more granular eGFR group cutoffs for the model containing DSP alone are reported in Supplemental Table 2A and for the model containing DSP and SG in Supplemental Table 2B.
Discussion
In this diverse cohort of 11,229 individuals, incorporating SG increased the predictive ability of DSP to accurately identify individuals with clinically significant proteinuria. From our model, we created an equation using urinalysis results that can be easily used to determine a patient's probability for having clinically significant proteinuria. We found that a DSP ≥300 virtually always indicates clinically significant proteinuria, a DSP of 30 or 100 is affected by SG but often indicative of clinically significant proteinuria, and a DSP of negative/trace is typically NS. We reported a cutoff of SG for each DSP category at which clinically significant proteinuria is likely, balancing the sensitivity and specificity, but depending on the clinical context, a more sensitive or specific cutoff may be warranted. This method can more accurately classify patients with a likely false-positive DSP because of concentrated urine and those who warrant further screening, which has important implications for identifying patients with early stage CKD in both clinical care and research settings. These patients can then be targeted for early interventions, such as initiation of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker and sodium-glucose cotransporter 2 inhibitor, to improve cardiovascular and kidney outcomes.
Prior studies have evaluated the accuracy of using DSP to identify clinically significant proteinuria.4–6,17,18 Most of these studies did not factor in the SG,4,5,17,18 and most prior studies used high proteinuria thresholds as their outcomes, such as UACR ≥300 mg/g, which were higher than the current guideline recommendations that we used in our study.1,19 One prior model incorporated both DSP and SG to identify patients with a UPCR ≥500 mg/g (0.5 g/g).6 They concluded that if a patient had a DSP value of trace or higher, the probability of overt proteinuria increased as SG decreased, but did not further quantify this relationship. We built on this concept by determining the SG cutoff at which each DSP value could identify clinically significant proteinuria and creating a clinically useful equation.
We also evaluated our model in several subgroups. There were no differences in sensitivity in subgroups by hypertension, diabetes, or eGFR. The specificity was slightly higher in those with an eGFR <60 compared with ≥60, but otherwise was not different between the subgroups. The model was highly specific in all subgroups, indicating low rates of false-positive results. Compared with a meta-analysis evaluating the estimation of UACR from DSP alone, our results showed similar sensitivity but higher specificity, likely because the inclusion of SG in our study excludes individuals with positive DSP due to a highly concentrated urine sample rather than the presence of clinically significant proteinuria.4 Differences in the PPV and NPV between subgroups were likely because of higher prevalence of clinically significant proteinuria in those with underlying diabetes, hypertension, or eGFR <60. The PLR and NLR, which do not depend on prevalence, only differed by eGFR subgroups, such that the PLR was higher and the NLR was lower in those with eGFR <60. Although the PPVs for DSP plus SG were modest for individuals with eGFR >60 ml/min per 1.73 m2, the NPVs were high, suggesting that individuals with preserved GFR will likely not have clinically significant proteinuria after a negative test result.
This study has several strengths. In addition to a large and diverse sample, we obtained clinical information to examine model performance in subgroups. We adapted our data to create clinically useful tools that can be used by providers and researchers. The formulas developed could be incorporated into the EHR to calculate the probability of clinically significant proteinuria using the commonly obtained urine dipstick protein and SG. Then, an EHR flag could automatically identify patients needing a quantitative measurement of proteinuria or for EHR-driven clinical research. The EHR could also be leveraged to reflexively order a UACR or UPCR for patients with a DSP and SG suggestive of clinically significant proteinuria.
There are also important limitations. We included a convenience EHR-derived sample, which may select for individuals at higher risk of proteinuria for whom the provider determined that a UACR or UPCR test was indicated. The study was not limited to individuals who had urine studies conducted on a single sample. Although we included diverse patients from a large urban academic center, this analysis was performed at a single site that reports the semiquantitative DSP results as negative, trace, 30, 100, or ≥300 mg/dl. Other centers may report DSP in different ways and the degree of standardization of these tests between laboratories is unclear, which would need to be separately evaluated and incorporated into our equation in future studies to expand the clinical implementability of our findings. Other factors can affect SG, such as hematuria, AKI, or a dilution/concentrating defect. The use of diagnosis codes to identify comorbidities leaves the possibility that some comorbidities may have been missed. Although we used ten-fold internal cross-validation, future studies should explore external validation of these results to improve generalizability, especially in targeted populations such as those with preserved eGFR.
In a large, diverse cohort of individuals with both a dipstick urinalysis and UPCR or UACR obtained within the same 24-hour period, adding the SG to the DSP accurately predicted clinically significant proteinuria using guideline-based definitions. The model performed well in important subpopulations of patients. We calculated a threshold of SG above which urine would be too concentrated for the DSP to indicate clinically significant proteinuria and developed an easy-to-use equation for providers, EHR developers, and researchers. These tools require only information from a dipstick urinalysis, allowing them to be easily used by clinicians to screen patients for proteinuria and by researchers to identify patients with early CKD who may not have otherwise received a quantified measurement of proteinuria.
Supplementary Material
Acknowledgments
Part of this research was presented as an oral abstract at the American Society of Nephrology Kidney Week national meeting in November 2022.
The content is solely the responsibility of the authors and does not represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the Veterans Affairs.
Disclosures
Disclosure forms, as provided by each author, are available with the online version of the article at http://links.lww.com/KN9/A501.
Funding
M.C. McAdams: National Institute of Diabetes and Digestive and Kidney Diseases (5T32DK007257-38). S.S. Hedayati: National Heart, Lung, and Blood Institute (R38HL150214) and Yin Quan-Yuen Distinguished Professorship in Nephrology at the University of Texas Southwestern Medical Center. L.P. Gregg: VA CSR&D Career Development Award (IK2CX002368) and Houston VA Health Services Research and Development Center for Innovations Grant (CIN13-413).
Author Contributions
Conceptualization: S. Susan Hedayati, Meredith C. McAdams.
Data curation: Vaishnavi Kannan, DuWayne L. Willett, Pin Xu.
Formal analysis: S. Susan Hedayati, Meredith C. McAdams, Pin Xu, Song Zhang.
Investigation: S. Susan Hedayati, Michael Li, Meredith C. McAdams, Pin Xu.
Methodology: L. Parker Gregg, S. Susan Hedayati, Meredith C. McAdams, Pin Xu.
Resources: S. Susan Hedayati.
Supervision: S. Susan Hedayati.
Validation: L. Parker Gregg, S. Susan Hedayati, Meredith C. McAdams, Pin Xu.
Visualization: Ella Carroll, L. Parker Gregg, S. Susan Hedayati, Michael Li, Meredith C. McAdams, Pin Xu.
Writing – original draft: Ella Carroll, L. Parker Gregg, S. Susan Hedayati, Michael Li, Meredith C. McAdams, Pin Xu.
Writing – review & editing: Ella Carroll, L. Parker Gregg, S. Susan Hedayati, Vaishnavi Kannan, Michael Li, Meredith C. McAdams, DuWayne L. Willett, Pin Xu.
Data Sharing Statement
Data cannot be shared. Data can be shared via request.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/KN9/A500.
Supplemental Table 1. LOINC used to identify UACR, UPCR, DSP, and specific gravity.
Supplemental Table 2A. Model performance for detecting proteinuria from DSP alone.
Supplemental Table 2B. Model performance for detecting proteinuria from DSP and specific gravity by eGFR categories.
Supplemental Figure 1. Increasing levels of DSP in univariable models and when adjusting for specific gravity were associated with clinically significant proteinuria. Circles represent unadjusted models and triangles represent models adjusted for specific gravity. As compared with unadjusted models, adjustment for specific gravity increased the odds ratios for clinically significant proteinuria at each level of DSP.
References
- 1.Group KDIGOKCW. KDIGO 2012 clinical practice guideline for evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1–150. doi: 10.1038/kisup.2012.73 [DOI] [PubMed] [Google Scholar]
- 2.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41(1):1–12. doi: 10.1053/ajkd.2003.50007 [DOI] [PubMed] [Google Scholar]
- 3.Ginsberg JM, Chang BS, Matarese RA, Garella S. Use of single voided urine samples to estimate quantitative proteinuria. N Engl J Med. 1983;309(25):1543–1546. doi: 10.1056/nejm198312223092503 [DOI] [PubMed] [Google Scholar]
- 4.Sumida K Nadkarni GN Grams ME, et al.; Chronic Kidney Disease Prognosis Consortium. Conversion of urine protein-creatinine ratio or urine dipstick protein to urine albumin-creatinine ratio for use in chronic kidney disease screening and prognosis: an individual participant-based meta-analysis. Ann Intern Med. 2020;173(6):426–435. doi: 10.7326/m20-0529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim D Lee DY Cho SH, et al. Diagnostic accuracy of urine dipstick for proteinuria in older outpatients. Kidney Res Clin Pract. 2014;33(4):199–203. doi: 10.1016/j.krcp.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Constantiner M Sehgal AR Humbert L, et al. A dipstick protein and specific gravity algorithm accurately predicts pathological proteinuria. Am J Kidney Dis. 2005;45(5):833–841. doi: 10.1053/j.ajkd.2005.02.012 [DOI] [PubMed] [Google Scholar]
- 7.Makihara N, Yamasaki M, Morita H, Yamada H. A dipstick test combined with urine specific gravity improved the accuracy of proteinuria determination in pregnancy screening. Kobe J Med Sci. 2011;56(4):E165–E172. PMID: 21937864 [PubMed] [Google Scholar]
- 8.Maschio G Alberti D Janin G, et al. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The angiotensin-converting-enzyme inhibition in progressive renal insufficiency study group. N Engl J Med. 1996;334(15):939–945. doi: 10.1056/nejm199604113341502 [DOI] [PubMed] [Google Scholar]
- 9.Ruggenenti P Perna A Gherardi G, et al. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet. 1999;354(9176):359–364. doi: 10.1016/s0140-6736(98)10363-x [DOI] [PubMed] [Google Scholar]
- 10.Heerspink HJL Stefánsson BV Correa-Rotter R, et al.; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 11.Sarraju A Li J Cannon CP, et al. Effects of canagliflozin on cardiovascular, renal, and safety outcomes in participants with type 2 diabetes and chronic kidney disease according to history of heart failure: results from the CREDENCE trial. Am Heart J. 2021;233:141–148. doi: 10.1016/j.ahj.2020.12.008 [DOI] [PubMed] [Google Scholar]
- 12.Norton JM Ali K Jurkovitz CT, et al. Development and validation of a pragmatic electronic phenotype for CKD. Clin J Am Soc Nephrol. 2019;14(9):1306–1314. doi: 10.2215/CJN.00360119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inker LA Eneanya ND Coresh J, et al.; Chronic Kidney Disease Epidemiology Collaboration. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–1749. doi: 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. doi: [DOI] [PubMed] [Google Scholar]
- 15.Neter J, Wasserman W, Kutner MH. Applied Linear Regression Models. Irwin; 1989. [Google Scholar]
- 16.Van Hoorde K, Van Huffel S, Timmerman D, Bourne T, Van Calster B. A spline-based tool to assess and visualize the calibration of multiclass risk predictions. J Biomed Inform. 2015;54:283–293. doi: 10.1016/j.jbi.2014.12.016 [DOI] [PubMed] [Google Scholar]
- 17.Mejia JR Fernandez-Chinguel JE Dolores-Maldonado G, et al. Diagnostic accuracy of urine dipstick testing for albumin-to-creatinine ratio and albuminuria: a systematic review and meta-analysis. Heliyon. 2021;7(11):e08253. doi: 10.1016/j.heliyon.2021.e08253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Usui T, Yoshida Y, Nishi H, Yanagimoto S, Matsuyama Y, Nangaku M. Diagnostic accuracy of urine dipstick for proteinuria category in Japanese workers. Clin Exp Nephrol. 2020;24(2):151–156. doi: 10.1007/s10157-019-01809-3 [DOI] [PubMed] [Google Scholar]
- 19.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 suppl 1):S1–S266. PMID: 11904577 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data cannot be shared. Data can be shared via request.