Abstract
Introduction
Bepirovirsen is a novel antisense oligonucleotide in development for chronic hepatitis B virus (HBV) infection therapy. Understanding the impact that clinical characteristics may have on bepirovirsen exposure is important for determining efficacious and well-tolerated dosing regimens. This analysis evaluated demographics and clinical characteristics associated with bepirovirsen exposure using a population pharmacokinetic (PK) analysis.
Methods
Population PK analyses were conducted using pooled data from three phase 1/2 clinical studies (NCT03020745/NCT02981602/NCT04449029) to construct a structural PK model for bepirovirsen that adequately described plasma concentration–time profiles and identify covariates that affect systemic exposure. The final population PK model was used to simulate bepirovirsen exposure measures to inform exposures at different dose levels and within different subpopulations.
Results
Bepirovirsen PK data were well-described by a linear, three-compartment model with first-order absorption and absorption delay. Chronic HBV infection status, body weight, and Asian versus non-Asian race were key covariates included in the final model. Visual inspection of correlation scatter plots confirmed general agreement between observed and predicted data from the studies. In simulations, bepirovirsen systemic exposure was dosed proportionally and predicted to be almost completely washed out by 12 weeks following the final 300-mg dose. Differences in body weight, Asian race, or disease status did not result in clinically relevant differences in exposure.
Conclusions
This analysis demonstrated that the linear three-compartmental model accurately described bepirovirsen PK data. The lack of clinically relevant differences seen in exposure indicate that dose adjustments are not recommended for bepirovirsen based on demographics or clinical characteristics.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-024-00980-9.
Keywords: Population pharmacokinetics, Chronic hepatitis B virus infection, Antisense oligonucleotide, Bepirovirsen
Plain Language Summary
Bepirovirsen is a drug in development for treating chronic hepatitis B virus (HBV) infection. Understanding how different characteristics impact the level of drug present in a patient’s body after they take a dose is useful when confirming how much of the drug to give, and how often, to ensure that the drug works and is well tolerated. Data from three previous clinical studies (NCT03020745/NCT02981602/NCT04449029) were used to create a model that described the level of bepirovirsen in patients over time and to identify any factors affecting this. This model was then used to simulate drug levels in patients to inform how levels change when different doses are used in patients with different characteristics. Bepirovirsen data were well described, with chronic HBV infection status, body weight, and Asian versus non-Asian race identified as key variables in the final model. Exposure to bepirovirsen increased with higher doses and was predicted to be almost completely cleared out of patients by 12 weeks following the final 300-mg dose. Differences in body weight, Asian race, or disease status (i.e., whether patients were infected with HBV or not) did not make any important changes to the level of bepirovirsen found in patients. This study showed that this model accurately described the levels of bepirovirsen present in different patients. As there were no important differences in the levels of drug in patients with different characteristics, the results suggest that there is no need to adjust treatment routines for bepirovirsen in patients with different characteristics.
Key Points
| Why carry out the study? |
| Bepirovirsen is a novel antisense oligonucleotide currently in development for the treatment of chronic hepatitis B virus (HBV) infection. |
| Previous studies have shown that bepirovirsen is effective in inducing sustained hepatitis B surface antigen and HBV deoxyribonucleic acid (DNA) loss in participants with chronic HBV infection. |
| The aim of this study was to characterize factors that may be associated with bepirovirsen exposure to inform treatment decisions such as dosing, timing, and patient selection. |
| What was learned from the study? |
| The developed population pharmacokinetic (PK) model accurately describes bepirovirsen PK systemic exposure and simulations, considering chronic HBV infection, body weight, and Asian race did not indicate clinically relevant differences in bepirovirsen exposure. |
| Results of this PK analysis suggest that no dose adjustment should be recommended for bepirovirsen based on demographic or baseline characteristics, or according to nucleos(t)ide analogue treatment status. |
| Information from this study will inform future trial design on patient population selection and dosing. |
Introduction
Chronic hepatitis B virus (HBV) infection is associated with a substantial global disease burden [1]. In 2019, it was estimated that 269 million people had chronic HBV infection globally, and there were 820,000 HBV-related deaths worldwide, mostly related to cirrhosis and liver cancer [1].
Functional cure is the endpoint endorsed by regulatory agencies and medical organizations for new chronic HBV infection therapies and is defined as sustained undetectable hepatitis B surface antigen (HBsAg) and HBV DNA in serum, with or without seroconversion of hepatitis B surface antibodies (anti-HBs), 24 weeks after completion of a finite course of treatment [2–5]. While existing therapies for chronic HBV infection, such as nucleos(t)ide analogs (NAs) and PEGylated interferon, can suppress HBV DNA and induce HBsAg loss, these therapies rarely achieve functional cure [2, 6, 7].
Bepirovirsen, a 2’-O-methoxyethyl novel, unconjugated antisense oligonucleotide (ASO), is in development for the treatment of chronic HBV infection. Phase 1/2 clinical trials have shown that bepirovirsen treatment induces HBsAg and HBV DNA loss, stimulates the immune system, and has an acceptable safety profile in participants with chronic HBV infection [8–10]. In fact, a recent phase 2b trial demonstrated that after 24 weeks of treatment with bepirovirsen 300 mg, 26% of participants on-NAs and 29% of participants not-on-NAs had an HBsAg level below the lower limit of quantification (LLOQ; 0.05 IU/ml) [9].
The absorption, distribution, metabolism, and excretion (ADME) of ASOs, such as bepirovirsen, is an evolving field. Adequate characterization of key pharmacokinetic (PK) attributes is critical for designing well-tolerated and efficacious dosing regimens for investigation in phase 3 clinical trials. A previous phase 1 study has shown that single and multiple doses of bepirovirsen at all dose levels (75–450 mg) were rapidly absorbed and distributed to peripheral tissues and slowly eliminated with little to no plasma accumulation over multiple doses [10].
This analysis investigated and assessed the possible effects of key patient characteristics (body weight, age, and ethnicity) on bepirovirsen exposure to inform treatment decisions, such as dosing and patient selection. A population PK analysis was performed to identify a structural PK model for bepirovirsen that adequately describes the plasma concentration–time profiles in healthy participants and participants with chronic HBV infection, estimate the population PK parameters for bepirovirsen, and identify potential covariates, including any effect of race, on relevant PK parameters.
Methods
Study Design
Data were pooled from three clinical studies (Supplementary Table 1) for which details have been published previously. Study 213725 (NCT03020745) was a phase 1, randomized, double-blind, placebo-controlled, dose-escalation study that assessed the safety, tolerability, and PK of bepirovirsen in healthy volunteers [10]. Participants were randomized 3:1 to receive bepirovirsen 75, 150, 300, or 450 mg (in sequential four-participant single- or multiple-dose cohorts) or placebo, subcutaneously, for up to 3 weeks. Study 205695 (NCT02981602) was a phase 2a, randomized, double-blind, placebo-controlled study that evaluated the efficacy, safety, and PK of bepirovirsen in participants with chronic HBV infection [8]. Participants were randomized 3:1 in each dose cohort to receive bepirovirsen (150 mg for participants Not-on-NAs or 300 mg for participants On-NAs or Not-on-NAs) or placebo subcutaneously for 4 weeks. Study 209668 (B-Clear; NCT04449029) was a phase 2b, multicenter, randomized, investigator-unblinded, parallel group study assessing the efficacy and safety of bepirovirsen in participants with chronic HBV infection either On-NAs or Not-on-NAs [9]. Participants were randomized 3:3:3:1 to receive once weekly: bepirovirsen 300 mg subcutaneously for 24 weeks; bepirovirsen 300 mg subcutaneously for 12 weeks then 150 mg for 12 weeks; bepirovirsen 300 mg subcutaneously for 12 weeks then placebo for 12 weeks; or placebo for 12 weeks then bepirovirsen 300 mg subcutaneously for 12 weeks.
Bepirovirsen concentrations in plasma were assayed using a validated hybridization enzyme-linked immunosorbent assay (ELISA) method (phase 1 study) or liquid–liquid extraction followed by high-performance liquid chromatography with tandem mass spectrometry detection (phase 2 studies). The LLOQ was 1 ng/ml for both methods.
All three studies were reviewed and approved by independent review boards, and written informed consent was obtained from all participants; all studies were conducted in accordance with the International Council on Harmonisation Guideline for Good Clinical Practice and the original principles embodied by the Declaration of Helsinki [8–10].
Data Transfer, Assembly, and Handling
Population PK analyses were conducted using nonlinear mixed effects modeling (NONMEM software version 7.4.4, ICON plc, Gaithersburg, MD, USA) interfaced with Finch Studio version ≥ 1.1.0 [11] and Perl-speaks-NONMEM version 5.2.6 [12]. SAS version 9.4 (SAS Institute, Inc., Cary, NC) was used for data preparation. R version 4.1.0 and R studio version 1.4.1717 were used for graphical analysis, model diagnostics, and output summaries. Simulations were conducted using mrgsolve (version 0.11.1). Actual dosing times and PK sampling times relative to the first dose were included in the time-ordered dataset, which was used in all analyses. Nominal PK sampling times were used in aggregate data displays where appropriate. Missing baseline covariates were imputed with the median value for continuous variables and with the most common value for discrete variables.
Base Structural Model Development
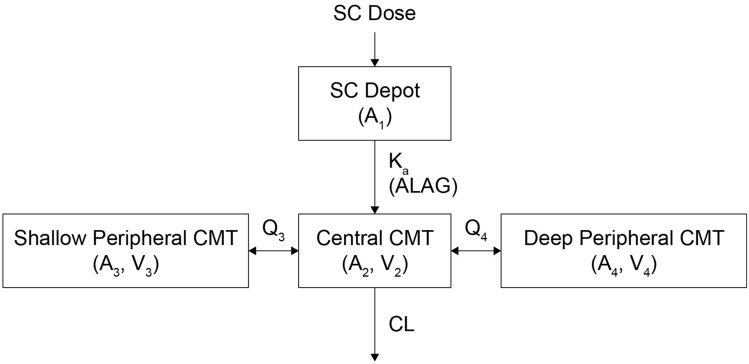
An exploratory graphical analysis of observed concentration–time data suggested that bepirovirsen PK is tri-exponential, with dose proportionality, and that participants with chronic HBV infection absorbed bepirovirsen more rapidly than healthy participants (Supplementary Fig. 1). Based on these previous observations and initial modeling attempts, a three-compartment model with the following features was used as the starting base model (Fig. 1): a first-order subcutaneous absorption from a depot compartment, an absorption delay into the central compartment, a distribution to and from the central compartment to two separate peripheral compartments, and linear elimination from the central compartment.
Fig. 1.
Schematic of the starting base three-compartment PK model after SC administration. A amount of drug in the compartment, ALAG absorption lag time, CL clearance, CMT compartment, Ka first-order absorption rate constant, PK pharmacokinetic, Q3 apparent intercompartmental clearance between central and shallow peripheral compartments, Q4 apparent intercompartmental clearance between central and deep peripheral compartments, SC subcutaneous, V volume of distribution
Estimated model parameters included first-order absorption rate constant (KA), absorption lag time (ALAG1), apparent clearance following extravascular administration (CL/F), apparent intercompartmental clearance between central and shallow (Q3/F) and central and deep (Q4/F) peripheral compartments, apparent central volume of distribution (V2/F), and apparent volume of distribution of the shallow (V3/F) and deep (V4/F) peripheral compartments.
Baseline body weight was included as covariate effect a priori on CL/F and V2/F, using a power model, based on previous exploratory analyses and initial modeling attempts. Iterations of the base model were explored with the weight effect power model exponents on CL/F and V2/F, both estimated as well as fixed to theoretical values (0.75 and 1 for CL/F and V2/F, respectively). Estimating weight exponents for CL/F and V2/F to be 0.48 and 0.92, respectively, resulted in the best minimum value of the NONMEM objective function (MVOF) and was statistically significantly better than no weight effect or fixed theoretical effect models (Supplementary Table 2).
Interindividual variability (IIV) was estimated for all parameters using exponential error models, assuming log-normal distribution. Residual variability (RV) for plasma bepirovirsen concentration–time data was estimated using a proportional error model and represents a composite of assay variability, intra-subject variability, model misspecification, error in timings of dosing and sampling, participant non-compliance, and other unaccounted factors. The equations used to calculate the IIV and RV are listed in the Supplementary Methods.
Assessment of Covariate Effects
Potential covariate effects were identified by graphical screening prior to stepwise selection. The relationships between random effects or individual error terms (ETAs) and the following pre-specified baseline continuous covariates of interest were analyzed by linear regression and visual inspection of correlation scatter plots for age, weight, serum creatinine, creatinine clearance, albumin, total bilirubin, and alanine aminotransferase (ALT). The relationships between ETAs and the following categorical covariates of interest, disease status (healthy vs. chronic HBV infection), sex, race, NA treatment status, and baseline HBsAg (> 1000 vs. ≤ 1000 IU/ml), were evaluated by visual inspection for differences between groups.
Covariates of clinical interest were included in the covariate search, regardless of whether they demonstrated correlation in the ETA-covariate plots. Analysis was conducted in a stepwise manner; covariates included in the stepwise search are shown in Supplementary Table 3. A univariate forward selection analysis was performed where covariates contributing a change of ≥ 6.635 in the minimum value of the NONMEM objective function (MVOF; level of significance [α] = 0.01, 1 degree of freedom [df]) were considered statistically significant and included in a full covariate model. The stepwise forward selection process was repeated until none of the remaining covariates produced a statistically significant reduction in MVOF. A univariate backward elimination was then performed where covariates contributing an increase of ≥ 10.827 in the MVOF (α = 0.001, 1 df) when removed from the model were considered statistically significant. The most non-significant covariate (highest p value > 0.001) was removed until all remaining covariates were statistically significant.
Model Evaluation and Discrimination Criteria
The models were assessed using multiple criteria, which were segregated into three groups as follows. The first was evaluation of individual and population mean PK parameter estimates and their precision by % relative standard error (RSE) of the population mean estimate and comparison of estimates to known prior or physiological values. Second was graphical examination of standard diagnostic and population analysis goodness-of-fit plots, including graphical examination of the agreement between the observed and individual post hoc predicted data over time or time since last dose (individual observed and predicted overlays). Finally, the models were also evaluated by measuring the reduction in both RV and IIV, and by comparing the MVOF for nested models.
Model Qualification
The final model was qualified for simulation by performing a dose cohort stratified prediction-corrected visual predictive check (pcVPC). This analysis graphically examined the agreement between the median and the 5th and 95th percentiles of the observed and simulated PK concentrations over time. Based on this graphical analysis, the model was refined as needed to correct for any substantial issues to the fixed or random effects parameters if discordance was seen between the observed and simulated data.
Population PK Model-Based Simulations
The final population PK model was used to simulate the following bepirovirsen exposure measures at steady state (1000 virtual participants per simulation): Cmax (maximum plasma concentration), Ctrough (trough plasma concentration), and AUCtau (area under the plasma bepirovirsen concentration–time curve during the dosing interval). Measures are presented by dose cohort and other relevant population stratifications including body weight, chronic HBV status, and Asian race versus non-Asian race.
Identification and Exclusion of Outliers
As outliers can negatively impact model convergence and/or final parameter estimates, they were excluded from the PK analysis. Visual inspection of individual and pooled PK data was predominantly used to identify outliers, and additional outliers were detected by graphical exploration of conditional weighted residuals (CWRES) during structural PK model development, and extreme individual post hoc parameter estimates. The final model was rerun after reintroduction of the excluded outliers into the analysis dataset as a sensitivity analysis.
Ethical Approval
Ethical approval was obtained for each study. The studies were conducted in accordance with the International Council on Harmonization Guideline for Good Clinical Practice and the Declaration of Helsinki. The study protocols, any amendments, informed consent, and other information that required pre-approval were reviewed and approved by a national, regional, or investigational center ethics committee or institutional review board, in accordance with the International Council for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice and applicable country-specific requirements, including US 21 Code of Federal Regulations (CFR) 312.3(b) for constitution of independent ethics committees. Each study obtained written informed consent from each participant prior to any study-specific procedures.
Results
Participants
In total, 516 patients were randomized across the three clinical studies (study 213725, n = 28; study 205695, n = 31; and study 209,668, n = 457), and 499 patients were eligible for this analysis. The majority of participants had chronic HBV infection (96%). The population was 63.7% male, 54.3% Asian, and 39.3% Caucasian. The mean (standard deviation [SD]) age was 45.4 (11.6) years, the mean (SD) weight was 71.2 (14.5) kg, and 47.5% had received concomitant NA treatment (Table 1). A total of 11,021 of 12,140 (90.8%) plasma bepirovirsen concentrations from 479 of 499 participants (96.0%) across the three clinical studies were used to develop the population PK model. Of the samples used, 525 (4.8%) plasma bepirovirsen concentrations were reported as below the LLOQ and were accommodated using the Beal M3 method.
Table 1.
Summary of demographics and baseline characteristics by study and overall
| Phase 1 study 213725 (N = 21) | Phase 2a study 205695 (N = 23) | Phase 2b study B-clear (N = 455) | Overall (N = 499) | |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 18 (85.7) | 11 (47.8) | 289 (63.5) | 318 (63.7) |
| Race, n (%) | ||||
| Asian | 0 (0.0) | 23 (100.0) | 248 (54.5) | 271 (54.3) |
| Black | 4 (19.0) | 0 (0.0) | 27 (5.9) | 31 (6.2) |
| Caucasian | 17 (81.0) | 0 (0.0) | 179 (39.3) | 196 (39.3) |
| Native Indian or Alaska Native | 0 (0.0) | 0 (0.0) | 1 (0.2) | 1 (0.2) |
| Age (years) | ||||
| Median [min, max] | 53.0 [27.0, 61.0] | 48.0 [18.0, 61.0] | 45.0 [18.0, 77.0] | 45.0 [18.0, 77.0] |
| Weighta(kg) | ||||
| Median [min, max] | 85.2 [62.0, 99.8] | 63.1 [43.9, 88.8] | 70.0 [43.3, 140.0] | 70.2 [43.3, 140.0] |
| Missing | 0 (0.0) | 0 (0.0) | 1 (0.2) | 1 (0.2) |
| NA treatment, n (%) | ||||
| No | 0 (0.0) | 18 (78.3) | 223 (49.0) | 241 (48.3) |
| Yes | 0 (0.0) | 5 (21.7) | 232 (51.0) | 237 (47.5) |
| NA | 21 (100.0) | 0 (0.0) | 0 (0.0) | 21 (4.2) |
| SCr (mg/dl) | ||||
| Median [min, max] | 0.9 [0.6, 1.2] | 0.7 [0.4, 1.1] | 0.8 [0.3, 1.3] | 0.8 [0.3, 1.3] |
| eGFR (ml/min/1.73 m2) | ||||
| Median [min, max] | 94.1 [70.1, 137.0] | 109.0 [64.1, 183.0] | 106.0 [65.8, 220.0] | 106.0 [64.1, 220.0] |
| CRCL (ml/min) | ||||
| Median [min, max] | 111.0 [81.9, 199.0] | 114.0 [51.7, 200.0] | 120.0 [59.9, 270.0] | 120.0 [51.7, 270.0] |
| Missing, n (%) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 1 (0.2) |
| Albumin (g/dl) | ||||
| Median [min, max] | 4.4 [4.1, 5.1] | 4.4 [4.0, 4.9] | 4.7 [3.8, 5.5] | 4.7 [3.8, 5.5] |
| Total bilirubin (mg/dl) | ||||
| Median [min, max] | 0.5 [0.3, 0.9] | 0.5 [0.2, 1.1] | 0.5 [0.2, 2.0] | 0.5 [0.2, 2.0] |
| ALT (IU/l) | ||||
| Median [min, max] | 17.0 [13.0, 44.0] | 30.0 [11.0, 107.0] | 24.0 [7.0, 349.0] | 24.0 [7.0, 349.0] |
| HBsAg (log10 IU/ml) | ||||
| Median [min, max] | – | 3.32 [1.75, 5.04] | 3.48 [1.64, 5.72] | 3.48 [1.64, 5.72] |
| Missing, n (%) | 21 (100.0) | 0 (0.0) | 0 (0.0) | 21 (4.2) |
ALT alanine transaminase, CRCL creatinine clearance, eGFR estimated glomerular filtration rate, HBsAg hepatitis B surface antigen, NA nucleos(t)ide analog, SCr serum creatinine
aOne participant was missing baseline weight, this value was imputed using the median value from the population
Covariate Selection
In step 1 of forward selection, the effect of disease status (chronic HBV infection versus healthy) on V3/F was the most statistically significant parameter–covariate relationship (p < 0.00001) (Supplementary Table 4). In step 2, the effect of disease status on ALAG1 was the most statistically significant parameter–covariate relationship (p = 0.00009) (Supplementary Table 4). Forward selection was concluded in step 3 (no statistically significant parameter–covariate relationships) (Supplementary Table 4). Backward elimination was concluded in step 1 as all covariate effects in the model were statistically significant (Supplementary Table 5).
Final Population PK Model
The final model was a linear three-compartmental model with first-order absorption and an absorption delay (lag time). Parameter estimates are presented in Table 2; all parameters were estimated with good precision (RSE < 20%). The exponent for the effect of weight on CL/F was 0.494 (less than theoretical exponent of 0.75), and on V2/F was 1.01 (similar to theoretical exponent of 1). IIVs (coefficient of variation [CV%]) were estimated to be 45.9% for KA, 38.2% for ALAG1, 31.7% for CL/F, 40.8% for V2/F, 51.2% for Q3/F, 146.0% for Q4/F, 75.2% for V3/F, and 198.0% for V4/F.
Table 2.
Parameter estimates of the final population PK model
| Model parameter | Description | Estimate | RSE, %a | IIV (CV%) | Shrinkage, % |
|---|---|---|---|---|---|
| CL/F (l/h) | Apparent systemic clearance | 3.10 | 2.89 | 0.096 (31.7) | 31.9 |
| WT on CL/F | Weight exponent on CL/F | 0.494 | 0.0237 | – | – |
| V2/F (L) | Apparent volume of distribution of the central compartment | 11.7 | 2.47 | 0.154 (40.8) | 54.6 |
| WT on V2/F | Weight exponent on V2/F | 1.01 | 0.0247 | – | – |
| KA (h–1) | Absorption rate constant | 0.237 | 3.48 | 0.192 (45.9) | 47.9 |
| ALAG1 (h), healthy | Absorption lag time | 0.232 | 4.68 | 0.136 (38.2) | 54.5 |
| Chronic HBV infection on ALAG1 | Proportional shift for chronic HBV infection on ALAG1 | – 0.466 | 8.40 | – | – |
| Q3/F (l/h) | Apparent intercompartmental clearance between central and shallow peripheral compartments | 0.107 | 2.91 | 0.233 (51.2) | 54.1 |
| V3/F (L), healthy | Apparent volume of distribution of the shallow peripheral compartment | 33.2 | 1.98 | 0.448 (75.2) | 41.8 |
| Chronic HBV infection on V3/F | Proportional shift for chronic HBV infection on V3/F | – 0.337 | 2.56 | – | – |
| Q4/F (l/h) | Apparent intercompartmental clearance between central and deep peripheral compartments | 0.0428 | 2.69 | 1.140 (146.0) | 25.8 |
| V4/F (L) | Apparent volume of distribution of the deep peripheral compartment | 63.7 | 2.25 | 1.590 (198.0) | 15.4 |
| RV (CV%) | CCV residual error | 0.0449 (21.2) | 1.59 | – | 0.0986 |
CL/F (l/h) = 3.10 · (WT/73)^0.494; V2/F (L) = 11.7 · (WT/73)^1.01; ALAG1 (h) = 0.232 (healthy participants); ALAG1 (h) = 0.232 * (1–0.466) (participants with chronic HBV infection); V3/F (l/h) = 33.2 (healthy participants); V3/F (l/h) = 33.2 * (1–0.337) (participants with chronic HBV infection)
ALAG1 absorption lag time, CCV constant coefficient of variation, CL/F apparent clearance following extravascular administration, CV coefficient of variation, HBV hepatitis B virus, IIV interindividual variability, KA first-order absorption rate constant, Q3/F apparent intercompartmental clearance between central and shallow peripheral compartments following extravascular administration, Q4/F apparent intercompartmental clearance between central and deep peripheral compartments following extravascular administration, RSE relative standard error, RV residual variation, V2/F apparent central volume of distribution following extravascular administration, V3/F apparent volume of distribution of the shallow peripheral compartments following extravascular administration, V4/F apparent volume of distribution of the deep peripheral compartments following extravascular administration, WT body weight
aReported RSE% are for the log-transformed parameter values
Goodness-of-fit plots demonstrated an unbiased and acceptable fit to the data (Supplementary Fig. 2). Visual inspection of pcVPC plots confirmed general agreement between the median and 5th and 95th percentiles for observed and predicted data for the phase 2b (300 mg weekly for 24 weeks data shown in Fig. 2), phase 2a, and phase 1 studies.
Fig. 2.
Population PK pcVPC by dose cohort and NA treatment status in the phase 2b study B-Clear (panels A and B show on-NA data; panels B and C show not-on-NA data). Observed data: individual (dots), 5th (dashed), median (solid), and 95th (dashed) percentile. Predicted data (shaded): 5th (lower blue), 50th (grey), and 95th (upper blue) prediction intervals and 95% CI around each of the prediction intervals. The probability of < LLOQ: solid line is fraction of observed data that is < LLOQ, and shaded area is 95% CI around predicted proportion. CI confidence interval, LLOQ lower limit of quantification, NA nucleos(t)ide analog, pcVPC prediction-corrected visual predictive check, PK pharmacokinetic
Simulated Bepirovirsen Exposure
Simulations demonstrated that bepirovirsen exposure increased in proportion to dose (Table 3). After 24 weeks of treatment with either bepirovirsen 300 mg or 450 mg, the median (90% confidence interval [CI]) for AUCtau ranged from 98.2 (57.6, 168.0) to 147.0 µg·h/ml (86.4, 252.0), for Cmax from 8.4 (4.2, 16.5) to 12.6 µg/ml (6.3, 24.8), and for Ctrough from 0.0224 (0.0113, 0.0459) to 0.0036 µg/ml (0.0170, 0.0689), respectively. Although chronic HBV infection was a significant covariate on ALAG and V3/F, bepirovirsen PK exposure did not differ by disease status (Table 4). Of the covariates included in the final model, body weight had the largest impact on the metrics AUCtau, Cmax, and Ctrough, with exposure decreasing with increased body weight. When body weight increased from 40 to 100 kg, the median (90% CI) for AUCtau decreased from 131.0 (78.4, 209.0) to 83.2 µg·h/ml (49.8, 133.0), Cmax reduced from 12.5 (6.3, 23.2) to 6.6 µg/ml (3.4, 11.9), and Ctrough from 0.0383 (0.0217, 0.0686) to 0.0160 µg/ml (0.0090, 0.0289). However, the ranges were not considered clinically relevant. Although race was not a statistically significant covariate, simulated bepirovirsen exposures were compared between Asian and non-Asian participants to assess differences in weight; the differences were not considered clinically relevant. Individual post hoc PK parameters and exposure metrics across races (Fig. 3; Supplementary Table 6; Supplementary Fig. 3) and regions (Supplementary Table 7; Supplementary Fig. 4) were also comparable.
Table 3.
Summary of population PK model-predicted exposures for bepirovirsen dosing regimens
| Dose group (no loading dose) | Time | AUCtau, µg·h/ml Median (90% CI) |
Cmax, µg/ml Median (90% CI) |
Ctrough, µg/ml Median (90% CI) |
|---|---|---|---|---|
| 450 mg × 24 weeks | Week 24 | 147.0 (86.4, 252.0) | 12.6 (6.3, 24.8) | 0.0336 (0.0170, 0.0689) |
| 300 mg × 24 weeks | Week 24 | 98.2 (57.6, 168.0) | 8.4 (4.2, 16.5) | 0.0224 (0.0113, 0.0459) |
| 300 mg × 12 weeks, then 150 mg × 12 weeks | Week 12 | 97.9 (57.4, 168.0) | 8.4 (4.2, 16.5) | 0.0205 (0.0106, 0.0419) |
| 300 mg × 12 weeks, then 150 mg × 12 weeks | Week 24 | 49.2 (28.9, 84.2) | 4.2 (2.1, 8.3) | 0.0120 (0.0059, 0.0254) |
N = 1000 participants included in each simulation
AUCtau area under the plasma bepirovirsen concentration–time curve during the dosing interval, CI confidence interval, Cmax maximum plasma bepirovirsen concentration, Ctrough trough plasma bepirovirsen concentration, PK pharmacokinetic
Table 4.
Summary of population PK model-predicted steady-state exposures for bepirovirsen 300 mg SC weekly for 24 weeks by covariates
| Covariate | AUCtau, µg·h/ml Median (90% CI) |
Cmax, µg/ml Median (90% CI) |
Ctrough, µg/ml Median (90% CI) |
|---|---|---|---|
| Population (disease status) | |||
| Healthy volunteer | 98.3 (57.6, 168.0) | 8.4 (4.2, 16.4) | 0.0243 (0.0126, 0.0492) |
| Patient with chronic HBV infection | 98.2 (57.6, 168.0) | 8.4 (4.2, 16.5) | 0.0224 (0.0113, 0.0459) |
| Weight | |||
| 40 kg | 131.0 (78.4, 209.0) | 12.5 (6.3, 23.2) | 0.0383 (0.0217, 0.0686) |
| 70 kg | 99.2 (59.4, 159.0) | 8.5 (4.4, 15.4) | 0.0225 (0.0126, 0.0404) |
| 100 kg | 83.2 (49.8, 133.0) | 6.6 (3.4, 11.9) | 0.0160 (0.0090, 0.0289) |
| Race (mean ± SD weighta) | |||
| Asian (65.2 ± 11.6 kg) | 102.0 (55.4, 160.0) | 8.9 (4.5, 17.3) | 0.0242 (0.0125, 0.0478) |
| Non-Asian (77.8 ± 14.8 kg) | 93.8 (55.4, 160.0) | 7.8 (4.0, 15.2) | 0.0205 (0.0105, 0.0413) |
N = 1000 participants included in each simulation
aObserved mean ± SD body weight in the phase 2b study B-Clear
AUCtau area under the plasma bepirovirsen concentration–time curve during the dosing interval, CI confidence interval, Cmax maximum plasma bepirovirsen concentration, Ctrough trough plasma bepirovirsen concentration, HBV hepatitis B virus, PK pharmacokinetic, SC subcutaneous SD standard deviation
Fig. 3.
PK exposure parameters stratified by race in the phase 2b study B-Clear. AUCtau area under the plasma bepirovirsen concentration–time curve during the dosing interval, Cmax maximum plasma bepirovirsen concentration, Ctrough trough plasma bepirovirsen concentration, PK pharmacokinetic
Simulated Bepirovirsen Concentrations Following End of Treatment
Bepirovirsen was predicted to be nearly completely washed out 12 weeks after the end of dosing. In participants who received bepirovirsen 300 mg weekly for 24 weeks, the median (95% CI) predicted concentration at week 36 was 93% lower than at the end of treatment (week 24: 0.0224 [0.0113, 0.0461] µg/ml versus week 36: 0.0017 [0.000048, 0.0102] µg/ml).
Sensitivity Analysis to Evaluate the Impact of Outlier Values
The fixed effects model parameters did not change significantly after the reintroduction of the excluded outliers. There were large changes in the random effect parameters with IIV CV% increasing by ≥ 42% in all parameters except KA, indicating a disproportionate influence of a small number of outliers on the IIV of the model parameters.
Discussion
This analysis of bepirovirsen data from healthy participants and participants with chronic HBV infection provides a valuable assessment of the PK of bepirovirsen across different baseline demographics and characteristics. Bepirovirsen PK data were well described by a linear, three-compartment model with first-order absorption and an absorption delay (lag time), the latter of which is often determined by drug transport and metabolism leading to fractional bioavailability of the drug [13]. In simulations, bepirovirsen exposure was comparable across patient characteristics, dose proportional, and predicted to be almost completely washed out by 12 weeks following the final dose of a 300 mg weekly for 24 weeks regimen.
In line with observations for other ASOs, bepirovirsen is expected to rapidly distribute into tissues and to be eliminated via nuclease-mediated metabolism in tissues and subsequent excretion of smaller oligonucleotide fragments in urine [10, 14–16]. This is consistent with the results of a phase 1, first-time-in-human study (NCT03020745), which showed a rapid decline in plasma concentration and a low level of renal elimination of full-length bepirovirsen over the first 24-h post dose, reflecting the distribution in tissues [10].
The PK of second-generation ASOs is typically described by a two-compartment model as in inotersen, danvatirsen, volanesorsen, and mipomersen, or a three-compartment model as in custirsen [17–21]. The PK parameter estimates are consistent across different ASOs, with clearance having been reported to be approximately 3 l/h. A two-compartment model was attempted for bepirovirsen, but the fitting was not adequate for the terminal phase.
The final model included the effect of body weight on CL/F and V2/F, and disease status (chronic HBV infection versus healthy) on V3/F and ALAG1. Exponents for the effect of weight on CL/F were 0.494, less than the theoretical exponent of 0.75, and on V2/F were 1.01 (similar to the theoretical exponent of 1), indicating that the relationship for apparent clearance following extravascular administration is less steep than the standard relationship for monoclonal antibodies. Of the included covariates, body weight had the largest impact on bepirovirsen exposures; however, the variation in exposure (difference in median AUCtau, Cmax, and Ctrough values of < 40% over a body weight range of 40–100 kg) is not considered clinically relevant; thus, no dose adjustment for body weight is recommended. Additionally, while disease status was found to be a significant covariate, the magnitude of effect of disease status on bepirovirsen exposure was not considered clinically relevant.
The population PK profile of bepirovirsen seen in this analysis aligns with that seen in other population PK studies of ASOs. A two-compartmental linear PK analysis of inotersen, a synthetic ASO for the treatment of hereditary transthyretin amyloidosis, demonstrated only a small effect of body weight or disease status on clearance and volume of distribution [17]. Similarly, a three-compartment model of custirsen, an ASO as adjunct to chemotherapy in patients with prostate or lung cancer, also demonstrated small but significant effects of body weight and age on clearance [19].
Variations in PK have been reported in different ethnic groups, including between Asian and non-Asian populations, with body weight playing a potential factor in these differences [22]. A key objective for this analysis was to assess the effect of Asian versus non-Asian race on bepirovirsen exposure. The data show that bepirovirsen exposures were comparable between Asian and non-Asian populations, with differences in median AUCtau, Cmax, and Ctrough values below 20%, which were not deemed clinically relevant. Furthermore, when assessing differences in weight, no statistically significant differences in exposure were observed, indicating that weight should not be highlighted as a potential source of inter-ethnic variation in the PK for the planning of future clinical trials for bepirovirsen and when assessing the suitability of the dosing regimen in Asian and non-Asian populations.
After accounting for weight and disease status, other demographic and baseline characteristics of clinical interest, baseline HBsAg level, age, and albumin, were not identified as PK covariates during formal covariate testing. Concomitant NA treatment was also not identified as a significant covariate, suggesting that NA treatment does not affect bepirovirsen PK. Participants with mild renal impairment were included in the analysis; however, mild renal impairment was also not identified as a covariate of bepirovirsen exposure in the population PK analysis. This indicates that no dose adjustment is required in participants with mild renal impairment.
This analysis was limited by the absence of measured liver concentrations, meaning that a semi-mechanistic population PK model that described the time course of the drug in the liver was not possible. Additionally, the studies used to inform the model did not include participants with cirrhosis/liver failure, moderate/severe chronic renal disease or other serious systemic disease, limiting generalizability of the results to these populations. Finally, there was no ability to evaluate the effect of anti-drug antibodies on bepirovirsen PK. The model will be updated using phase 3 data, which will include anti-drug antibody data.
Conclusions
In the current analysis, we developed a linear three-compartmental model that accurately described bepirovirsen PK as a representation of ASO disposition. There were no clinically relevant differences in bepirovirsen exposure across covariates, and bepirovirsen was predicted to be nearly completely washed out 12 weeks after the end of dosing. These results suggest no dose adjustment should be recommended for bepirovirsen based on demographic or baseline characteristics or upon coadministration with NAs. The developed population PK model will form the basis for future PK/pharmacodynamic modeling to describe bepirovirsen effects on HBsAg levels in patients with chronic HBV infection.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the participants and their families, clinicians, and study investigators.
Medical Writing/Editorial Assistance
Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables and figures, grammatical editing, and referencing) was provided by Ciara Keogh, PhD, of Fishawack Indicia Ltd. (part of Avalere Health), and was funded by GSK.
Author contributions
The authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors, take responsibility for the integrity of the work as a whole, contributed to the writing and reviewing of the manuscript, and have given final approval for the version to be published. Ahmed Nader, Amir S Youssef, Kelong Han, and Mindy Magee were involved in the conception and design of the study. Ahmed Nader, Amir S Youssef, Kelong Han, Mindy Magee, and Mohamed Ismail were involved in data analysis and interpretation.
Funding
This study was funded by GSK (GSK Study ID 218363). The study sponsor also funded the journal’s Rapid Service Fee.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
Ahmed Nader, Amir S Youssef, Kelong Han, and Mindy Magee are employees of GSK and hold stocks/shares in GSK. Mohamed Ismail is an employee of Enhanced Pharmacodynamics LLC and clinical pharmacology and pharmacometrics consultants supporting EOP2 analysis.
Ethical approval
Ethical approval was obtained for each study. The studies were conducted in accordance with the International Council on Harmonization Guideline for Good Clinical Practice and the Declaration of Helsinki. The study protocols, any amendments, informed consent, and other information that required pre-approval were reviewed and approved by a national, regional, or investigational center ethics committee or institutional review board, in accordance with the International Council for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice and applicable country-specific requirements, including US 21 Code of Federal Regulations (CFR) 312.3(b) for constitution of independent ethics committees. Each study obtained written informed consent from each participant prior to any study-specific procedures.
Footnotes
The original online version of this article was revised due to typographic error in the paragraph a typographic error in the paragraph below Fig 2 caption, where the unit in the last two lines should read microgram/ml instead of nanogram/ml.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
10/26/2024
A Correction to this paper has been published: 10.1007/s40121-024-01066-2
References
- 1.World Health Organization. Hepatitis B factsheet 2023. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b.
- 2.Lok AS, Zoulim F, Dusheiko G, Ghany MG. Hepatitis B cure: from discovery to regulatory approval. Hepatology. 2017;66(4):1296–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song A, Lin X, Chen X. Functional cure for chronic hepatitis B: accessibility, durability, and prognosis. Virol J. 2021;18(1):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghany MG, Buti M, Lampertico P, Lee HM, AASLD-EASL HBV-HDV Treatment Endpoints Conference Faculty. Guidance on treatment endpoints and study design for clinical trials aiming to achieve cure in chronic hepatitis B and D: report from the 2022 AASLD-EASL HBV/HDV treatment endpoints conference. Hepatology. 2023. [DOI] [PMC free article] [PubMed]
- 5.Food and Drug Administration. Chronic hepatitis B virus infection: developing drugs for treatment guidance for industry 2022. https://www.fda.gov/media/117977/download.
- 6.Loglio A, Lampertico P. How durable is functional cure (hepatitis B surface antigen loss) in patients with chronic hepatitis b treated with current antivirals? Hepatol Commun. 2020;4(1):5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seto WK, Lo YR, Pawlotsky JM, Yuen MF. Chronic hepatitis B virus infection. Lancet. 2018;392(10161):2313–24. [DOI] [PubMed] [Google Scholar]
- 8.Yuen MF, Heo J, Jang JW, Yoon JH, Kweon YO, Park SJ, et al. Safety, tolerability and antiviral activity of the antisense oligonucleotide bepirovirsen in patients with chronic hepatitis B: a phase 2 randomized controlled trial. Nat Med. 2021;27(10):1725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuen MF, Lim SG, Plesniak R, Tsuji K, Janssen HLA, Pojoga C, et al. Efficacy and safety of bepirovirsen in chronic hepatitis B infection. N Engl J Med. 2022;387(21):1957–68. [DOI] [PubMed] [Google Scholar]
- 10.Han K, Theodore D, McMullen G, Swayze E, McCaleb M, Billioud G, et al. Preclinical and phase 1 assessment of antisense oligonucleotide bepirovirsen in hepatitis B virus-transgenic mice and healthy human volunteers: support for clinical dose selection and evaluation of safety, tolerability, and pharmacokinetics of single and multiple doses. Clin Pharmacol Drug Dev. 2022;11(10):1191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ismail M, Van Wart S, Mager DE. Abstr 10062. Finch Studio: a next generation NONMEM modeling workbench. PAGE 302022.
- 12.Lindbom L, Pihlgren P, Jonsson EN. PsN-Toolkit–a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Prog Biomed. 2005;79(3):241–57. [DOI] [PubMed] [Google Scholar]
- 13.Koch G, Krzyzanski W, Pérez-Ruixo JJ, Schropp J. Modeling of delays in PKPD: classical approaches and a tutorial for delay differential equations. J Pharmacokinet Pharmacodyn. 2014;41(4):291–318. [DOI] [PubMed] [Google Scholar]
- 14.Geary RS. Antisense oligonucleotide pharmacokinetics and metabolism. Expert Opin Drug Metab Toxicol. 2009;5(4):381–91. [DOI] [PubMed] [Google Scholar]
- 15.Geary RS, Norris D, Yu R, Bennett CF. Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv Drug Deliv Rev. 2015;87:46–51. [DOI] [PubMed] [Google Scholar]
- 16.Geary RS, Yu RZ, Watanabe T, Henry SP, Hardee GE, Chappell A, et al. Pharmacokinetics of a tumor necrosis factor-alpha phosphorothioate 2′-O-(2-methoxyethyl) modified antisense oligonucleotide: comparison across species. Drug Metab Dispos. 2003;31(11):1419–28. [DOI] [PubMed] [Google Scholar]
- 17.Yu RZ, Collins JW, Hall S, Ackermann EJ, Geary RS, Monia BP, et al. Population pharmacokinetic–pharmacodynamic modeling of inotersen, an antisense oligonucleotide for treatment of patients with hereditary transthyretin amyloidosis. Nucl Acid Ther. 2020;30(3):153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu H, Tong X, Mugundu G, Scott ML, Cook C, Arfvidsson C, et al. Population pharmacokinetic analysis of danvatirsen supporting flat dosing switch. J Pharmacokinet Pharmacodyn. 2019;46(1):65–74. [DOI] [PubMed] [Google Scholar]
- 19.Edwards AY, Elgart A, Farrell C, Barnett-Griness O, Rabinovich-Guilatt L, Spiegelstein O. A population pharmacokinetic meta-analysis of custirsen, an antisense oligonucleotide, in oncology patients and healthy subjects. Br J Clin Pharmacol. 2017;83(9):1932–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Food and Drug Administration. Center for drug evaluation and research clinical pharmacology and biopharmaceutics review—Mipomersen. FDA; 2012.
- 21.European Medicines Agency. Assessment report—Volanesorsen. EMA; 2019.
- 22.Olafuyi O, Parekh N, Wright J, Koenig J. Inter-ethnic differences in pharmacokinetics-is there more that unites than divides? Pharmacol Res Perspect. 2021;9(6): e00890. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.