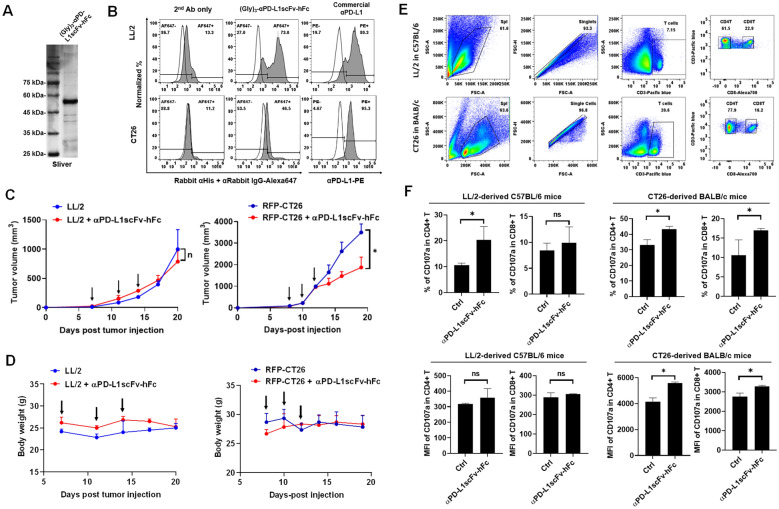
Fig. 7.
CT26 exhibits αPD-L1scFv-hFc immunotherapy-sensitive but LL/2 exhibits resistant. A αPD-L1scFv-h(human)Fc was constructed and purified by Ni-column and detected using silver staining. B Flow cytometry was used to detect the binding capacity of αPD-L1scFv-hFc with LL/2 and CT26 cells in vitro, whereas a commercial αPD-L1 was used as a positive control. C The LL/2 and CT26 cells were subcutaneously injected into their syngeneic mice, BALB/c (n = 3) and C57BL/6 (n = 3), respectively. Individual 100 μg of αPD-L1scFv-hFc was administrated intraperitoneally on day 7, 11, and 14 for LL/2-derived mice and on day 8, 10, and 12 for CT26-derived mice (indicated by arrows). D Body weights were documented to be used as a toxicity marker in αPD-L1scFv-hFc administration. E Splenocytes harvested after the end of tumor size measurement from LL/2-derived C57BL/6 (n = 3) and CT26-derived BALB/c mice (n = 3) were analyzed using flow cytometry to F detect the activation marker CD107a expression in T lymphocytes, including CD4+ T and CD8+ T cells. *p < 0.05