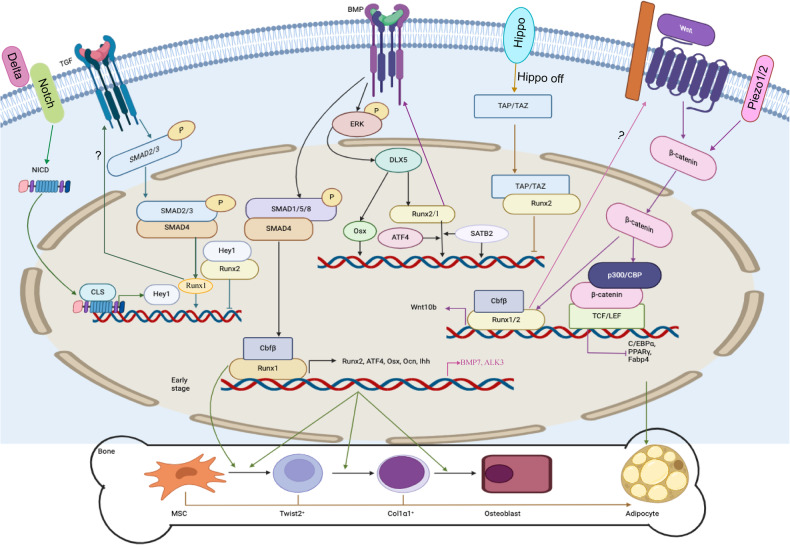
Fig. 4. Signaling and transcriptional regulation of osteoblast cell lineage commitment, differentiation, and bone formation.
Among the many transcription factors that participate in osteoblast differentiation, the Runx family, Osx, ATF4, Cbfβ, and SATB2 are significant. Cbfβ binds to Runx family proteins to form heterodimers, improving Runx2’s and Runx1’s stability and subsequently facilitating Runx2 or Runx1 binding to target DNA sequences. Runx1 positively regulates osteoblast lineage gene expression at various stages of differentiation. Runx1 plays a significant role in postnatal bone homeostasis by binding to ATF4, Ocn, and Runx2 promoters to activate the corresponding genes and promote osteoblast early differentiation. Runx1 promotes BMP7 and Alk3 expression to regulate BMP signaling. How Runx1 can regulate Wnt10b and TGF-β signaling remains unclear. Runx1 can also regulate osteoblast-adipocyte lineage via inducing Wnt/β-catenin signaling, TGF-β signaling, and restraining adipogenic gene transcription. Cbfβ is also crucial in stimulating osteogenesis by inhibiting the expression of the adipogenesis regulatory gene C/EBPα and activating Wnt10b/β-catenin signaling. Wnt binds with FZD receptors, causing the ß-catenin accumulation. Pizeo1/2 can also regulate ß-catenin. ß-catenin then moves to the nucleus, in which it causes target genes to be transcribed by interacting with P300/CBP and TCF/LEF. TGF-β and BMP regulate transcription factors through SMAD proteins to activate Runx1 and Runx2 activity, and the SMAD itself can also regulate osteoblast-specific gene expression. BMP signaling can also activate Dlx5 through ERK and promote Runx2, ATF4, and Osx expression. When the Hippo signaling is at the “off” state, YAP/TAZ can translocate into the nucleus and bind with Runx2 to inhibit its activity. NICD1 in Notch signaling can translocate into the nucleus and promote the expression Hey1, which in turn inhibits Runx2 function by binding with Runx2. The activation of β-catenin and Runx2 will also inhibit the expression of C/EBPα, PPAR-γ, and Fabp4; these genes are important in adipocyte differentiation.