Key Clinical Message
We encountered an extremely low birth weight infant with breast milk‐transmitted cytomegalovirus (CMV) infection. To determine the transmission route, we conducted direct sequence analysis of two variable CMV genes, UL139, and UL146. When utilizing breast milk, the possibility of acquired CMV infection should be considered and tested for prompt diagnosis and treatment.
Keywords: breast milk, cytomegalovirus, infection, preterm infant
1. INTRODUCTION
It is reported that cytomegalovirus (CMV) can be transmitted to newborns through breast milk, other CMV‐infected individuals, or blood transfusion products. In preterm infants, it can occasionally progress to severe CMV disease. Moreover, acquired infections in infants during the neonatal period have been reported to impact the development of cognitive or motor functions later in life. 1 , 2 , 3 , 4 , 5 , 6 Consequently, it is important to identify the route of infection, prevent CMV transmission, and intervene early when patients are diagnosed. For precise diagnosis of breast milk‐transmitted CMV (BM‐CMV) or transfusion‐transmitted CMV (TT‐CMV), analyzing the sequences of two CMV genes, UL139, and UL146, which are known for sequence variation among different strains, is helpful. 7 , 8 , 9
Here we present a case involving an extremely low birth weight (ELBW) infant with acquired BM‐CMV infection and describe the results of sequence analysis of two variable CMV genes in the patient's urine, frozen breast milk, and repository blood samples corresponding to transfused leukoreduced red blood cells (LR‐RBCs).
2. CASE HISTORY
A female infant weighing 784 g without signs of congenital CMV infection on fetal screening echocardiography was born at 25 weeks of gestation. Analysis of her mother's blood, conducted 1 week before the cesarean section, revealed positivity for anti‐CMV immunoglobulin (Ig) G, although she tested negative for anti‐CMV IgM. The patient's anti‐CMV IgM test results at birth were also negative. The patient started feeding on breast milk that was fresh on day 1 and fully transitioned to frozen breast milk after a few days. On days 7 and 8, the patient received a transfusion of 16 mL of LR‐RBCs for improvement in hemodynamics. Given the urgency, CMV‐undetermined LR‐RBCs were used instead of a CMV‐seronegative supply. Oral feeding was established on day 70, and direct breastfeeding commenced on day 78. Fundus hemorrhage was detected on day 84, and purpura appeared on the forearm on day 85.
On day 92, laboratory examinations revealed increased atypical lymphocytes (10% of white blood cells [WBCs]) and decreased platelets (10.4 × 103/μL) as well as low IgG levels (71 mg/dL). Additionally, the patient tested positive for anti‐CMV IgM and the CMV antigen (65/41 cells/50,000 peripheral blood WBCs); this indicated established CMV infection. Oral treatment with valganciclovir (VGCV; 32 mg/kg/day) was initiated on day 98. Following treatment, her clinical symptoms disappeared and laboratory parameters improved, with no further recurrence (Figure 1). Magnetic resonance imaging on day 86 and an auditory brainstem response test on day 100 revealed no abnormalities.
FIGURE 1.
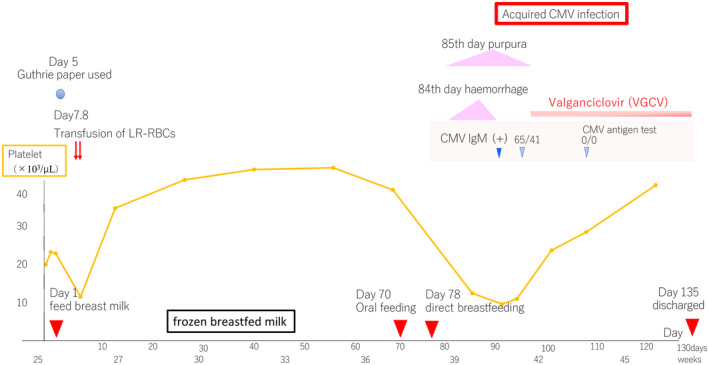
Progress chart.The horizontal axis shows age in days, and the vertical axis shows platelet count.
3. METHODS
The Guthrie paper, initially used on day 5 to screen for metabolic abnormalities, was frozen and utilized for CMV‐polymerase chain reaction testing, yielding negative results. The diagnosis was acquired CMV infection. Initially suspecting TT‐CMV, we commissioned The Japanese Red Cross Society to analyze the sequences of two variable CMV genes, UL139 and UL146, via direct sequence analysis of samples from the patient's urine, the mother's frozen breast milk, and polyclonal CMV strains in transfused LR‐RBCs. The sequence of the hypervariable region of the CMV strain detected in both urine and frozen breast milk was a perfect match, confirming BM‐CMV. Breastfeeding continued after the family was informed of the situation. The patient's mother was not rechecked for the CMV antigen and CMV IgM after delivery to confirm recent active infection.
4. CONCLUSION AND RESULTS
The patient was discharged on day 135, managed on an outpatient basis, and treated with VGCV until the corrected age of 1 year, when the antigen test turned positive and prompted cessation of treatment. Screening for various immunodeficiency diseases revealed no abnormal findings. After treatment, the patient displayed no developmental problems. At the time of writing this report, she was 1 year and 6 months old and healthy. Nevertheless, long‐term developmental follow‐up is essential.
5. DISCUSSION
In preterm infants, who often receive both blood transfusions and breast milk, the source of transmitted CMV should be definitively identified. The prevalence of BM‐CMV is considerably higher than what was previously believed. Seed et al. reported an extremely low risk of TT‐CMV with the use of leukoreduced blood products, 10 and Josephson et al. recommended CMV‐seronegative transfusions. 11
Josephson et al. also reported that the risk factors for BM‐CMV infection include the number of breast milk feeding days, viral load in breast milk, and premature rupture of membranes. 11 In the present case, premature rupture of membranes was not observed, but the patient was fully breastfed. A previous study indicated a significant increase in the viral load of CMV, which peaked 4–5 weeks later, in the breast milk of a transmitter after delivery. 12 In this case, the frozen breast milk of the mother was tested on day 100, well beyond the reported peak period. These findings suggest highly transmittable CMV in the breast milk in this case.
In conclusion, the benefits of breast milk for preterm infants are deemed so high that the American Academy of Pediatrics asserts the following: “The value of routinely feeding fresh human milk from CMV seropositive mothers to preterm infants outweighs the risks of clinical disease, especially because no long‐term neurodevelopmental abnormalities have been reported.” 13 However, in this case, frozen breast milk did not prevent CMV transmission. Therefore, when utilizing breast milk, the possibility of acquired CMV infection should be considered and tested for prompt diagnosis and treatment.
AUTHOR CONTRIBUTIONS
Tamao Shinohara: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; resources; software; supervision; validation; visualization; writing – original draft; writing – review and editing. Atsushi Nemoto: Conceptualization; data curation; supervision; writing – review and editing. Hiroyuki Fujihara: Conceptualization; data curation; writing – review and editing. Yasushi Murakami: Conceptualization; data curation; writing – review and editing. Yuki Maebayashi: Conceptualization; writing – review and editing. Tomohiro Saito: Writing – review and editing. Nobuyuki Katsumata: Conceptualization; writing – review and editing. Atsushi Naitoh: Conceptualization; writing – review and editing.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Informed consent was obtained from the patient's parents.
CONSENT
Written informed consent was obtained from the patient's parents for the publication of this case report and any accompanying images. A copy of the written consent form is available for review by the Chief Editor of the Journal.
ACKNOWLEDGMENTS
We thank the Japanese Red Cross Society, Yamanashi, Japan, for the data analysis. Additionally, we want to thank the members of the Department of Neonatology.
Shinohara T, Nemoto A, Fujihara H, et al. Breast milk‐transmitted acquired cytomegalovirus infection in an extremely low birth weight infant. Clin Case Rep. 2024;12:e9127. doi: 10.1002/ccr3.9127
DATA AVAILABILITY STATEMENT
All information pertaining to this case is included in this case report.
REFERENCES
- 1. Brecht KF, Goelz R, Bevot A, Krägeloh‐Mann I, Wilke M, Lidzba K. Postnatal human cytomegalovirus infection in preterm infants has long‐term neuropsychological sequelae. J Pediatr. 2015;166(4):834‐839.e1. doi: 10.1016/j.jpeds.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 2. Omarsdottir S, Casper C, Navér L, et al. Cytomegalovirus infection and neonatal outcome in extremely preterm infants after freezing of maternal milk. Pediatr Infect Dis J. 2015;34(5):482‐489. doi: 10.1097/INF.0000000000000619 [DOI] [PubMed] [Google Scholar]
- 3. Goelz R, Meisner C, Bevot A, Hamprecht K, Kraegeloh‐Mann I, Poets CF. Long‐term cognitive and neurological outcome of preterm infants with postnatally acquired CMV infection through breast milk. Arch Dis Child Fetal Neonatal Ed. 2013;98(5):F430‐F433. doi: 10.1136/archdischild-2012-303384 [DOI] [PubMed] [Google Scholar]
- 4. Lanzieri TM, Dollard SC, Josephson CD, Schmid DS, Bialek SR. Breast milk‐acquired cytomegalovirus infection and disease in VLBW and premature infants. Pediatrics. 2013;131(6):e1937‐e1945. doi: 10.1542/peds.2013-0076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bevot A, Hamprecht K, Krägeloh‐Mann I, Brosch S, Goelz R, Vollmer B. Long‐term outcome in preterm children with human cytomegalovirus infection transmitted via breast milk. Acta Paediatr. 2012;101(4):e167‐e172. doi: 10.1111/j.1651-2227.2011.02538.x [DOI] [PubMed] [Google Scholar]
- 6. Jim WT, Shu CH, Chiu NC, et al. Transmission of cytomegalovirus from mothers to preterm infants by breast milk. Pediatr Infect Dis J. 2004;23(9):848‐851. doi: 10.1097/01.inf.0000137571.35541.55 [DOI] [PubMed] [Google Scholar]
- 7. Furui Y, Yamagishi N, Morioka I, et al. Sequence analyses of variable cytomegalovirus genes for distinction between breast milk‐ and transfusion‐transmitted infections in very‐low‐birth‐weight infants. Transfusion. 2018;58(12):2894‐2902. doi: 10.1111/trf.14920 [DOI] [PubMed] [Google Scholar]
- 8. Yamagishi N, Furui Y, Koshinami S, et al. Sequence analysis of two variable cytomegalovirus genes for distinction between transfusion‐ and breast milk‐transmitted infections in a very‐low‐birthweight infant. Transfusion. 2016;56(6):1305‐1310. doi: 10.1111/trf.13547 [DOI] [PubMed] [Google Scholar]
- 9. Bradley AJ, Kovács IJ, Gatherer D, et al. Genotypic analysis of two hypervariable human cytomegalovirus genes. J Med Virol. 2008;80(9):1615‐1623. doi: 10.1002/jmv.21241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seed CR, Wong J, Polizzotto MN, Faddy H, Keller AJ, Pink J. The residual risk of transfusion‐transmitted cytomegalovirus infection associated with leucodepleted blood components. Vox Sang. 2015;109(1):11‐17. doi: 10.1111/vox.12250 [DOI] [PubMed] [Google Scholar]
- 11. Josephson CD, Castillejo MI, Caliendo AM, et al. Prevention of transfusion‐transmitted cytomegalovirus in low‐birth weight infants (≤1500 g) using cytomegalovirus‐seronegative and leukoreduced transfusions. Transfus Med Rev. 2011;25(2):125‐132. doi: 10.1016/j.tmrv.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Josephson CD, Caliendo AM, Easley KA, et al. Blood transfusion and breast milk transmission of cytomegalovirus in very low‐birth‐weight infants: a prospective cohort study. JAMA Pediatr. 2014;168(11):1054‐1062. doi: 10.1001/jamapediatrics.2014.1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnston M, Landers S, Noble L, Szucs K, Viehmann L. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827‐e841. doi: 10.1542/peds.2011-3552 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All information pertaining to this case is included in this case report.


