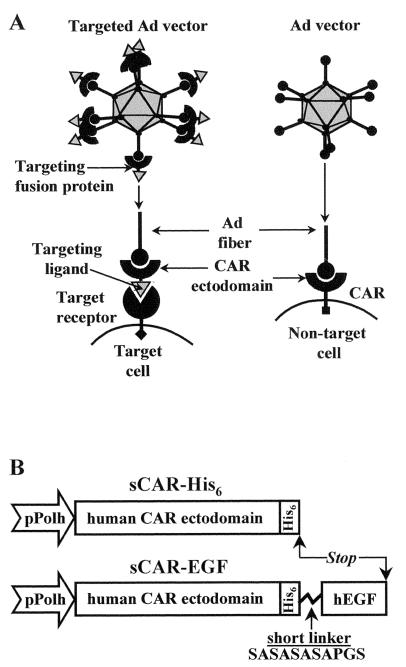
FIG. 1.
(A) Utilization of sCAR-ligand fusion proteins for receptor-specific targeting of Ad vectors. Ad vectors normally achieve cell binding via interaction between the knob domain of viral fiber protein with CAR. To redirect an Ad vector to an alternative cell surface receptor, a genetically engineered targeting fusion protein consisting of the CAR ectodomain fused to a receptor-specific targeting ligand was used. By virtue of its dual binding capacity, this complex serves as a bridge between an Ad virion and a cell-specific receptor molecule, thereby providing novel cell-binding capacity to the virion. (B) Construction of sCAR fusion proteins. The gene coding for either human sCAR-His6 or sCAR-EGF was constructed in a baculovirus expression vector. Expression is driven from the polyhedrin promoter (pPolh). A His6 tag was introduced for purification purposes into the carboxy terminus of the extracellular CAR domain (236 aa). To construct sCAR-EGF protein, human EGF (53 aa) was fused with the CAR ectodomain by a flexible linker (SASASASAPGS) and tagged with His6. See Materials and Methods for details of construction.