Figure 2.
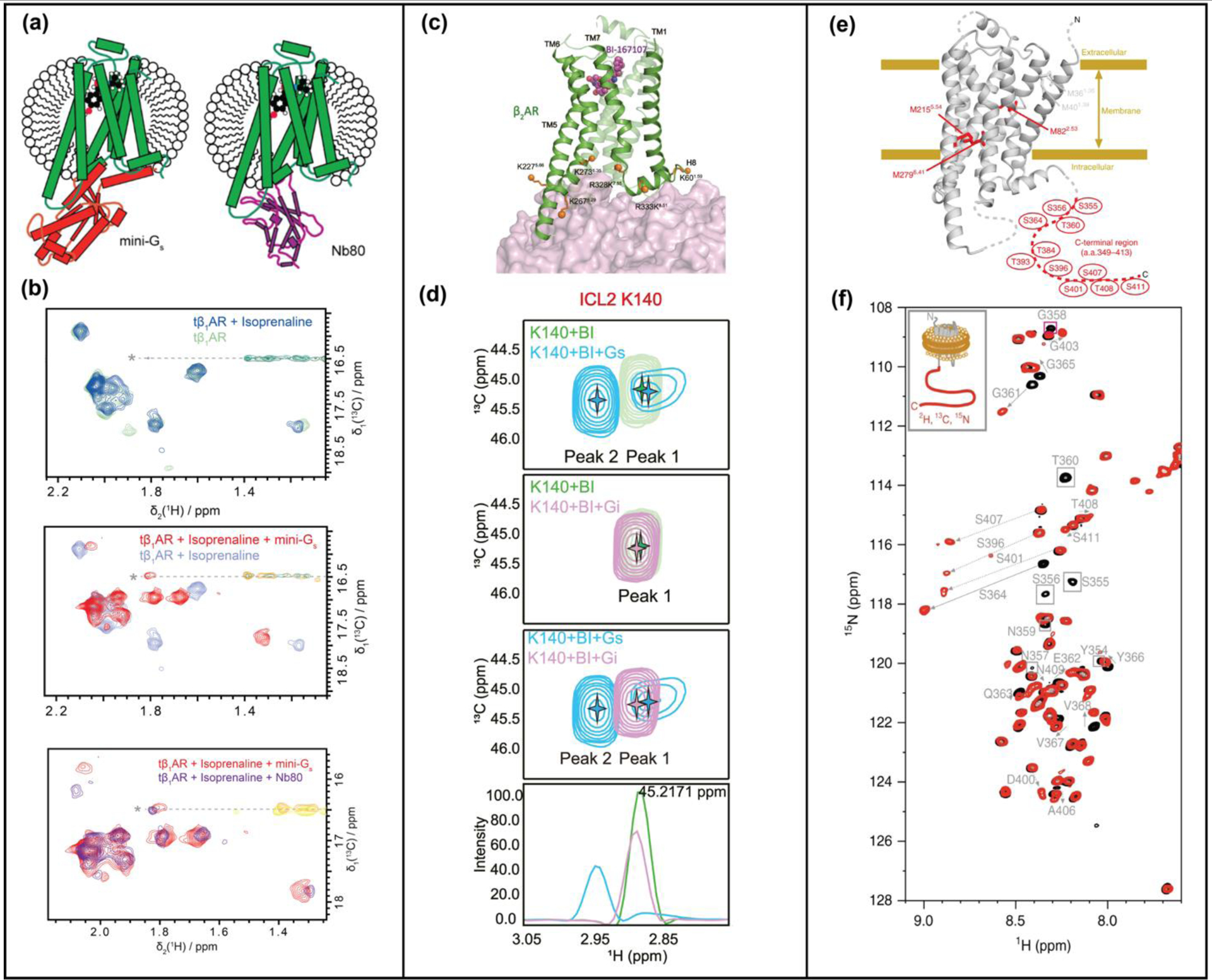
NMR studies of GPCR ternary complexes. (a) Complexes of tβ1AR with an engineered Gs protein (mini-Gs) and G protein-mimicking nanobody, Nb80, compared by NMR. Schematics show β1AR ternary complexes with an agonist and mini-Gs or nanobody NB80. (b) Superimposed [13C,1H]-HMQC spectra of β1AR in complex with an agonist and in ternary complexes with either mini-GS or Nb80. (c) β2AR-G protein interactions studied by reductive methylation of lysines. Lysines used as NMR probes are shown as orange solid spheres on the structure of the β2AR-GS complex (PDB 3SN6). (d) Expanded panels from [13C,1H]-HSQC spectra of β2AR highlighting chemical shift differences of K140between complexes with GS and Gi, indicating involvement of ICL2 in differentiating GS and Gi. (e) Phosphorylated β2AR and β2AR in complex with arrestin studied with ε−13CH3-methionine labeling and segmental labeling. (f) Superimposed [15N,1H]-HSQC spectra of β2AR (black) and phosphorylated β2AR (red) with segmentally-labeled C-terminus. Significant chemical shift changes are indicated with arrows. Panels a and b adapted from reference 17, c and d adapted from reference 19, and e and f adapted from reference 23, with permission.
