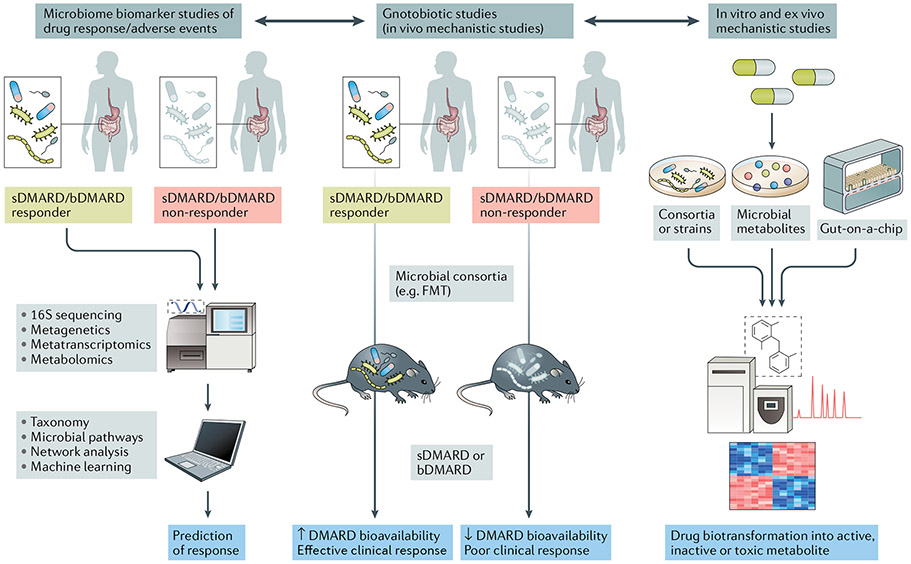
Fig. 3 ∣. Translational implications of pharmacomicrobiomic studies in rheumatic diseases.
In clinical studies with deeply phenotyped patient populations and known outcomes of synthetic (sDMARDs) or biologic DMARDs (bDMARDs) (such as efficacy and adverse events) (left panel), microbial features can be integrated with established biomarkers of response (for example, host genetics or immune cell profiles) via machine learning and network analyses to develop predictive tools. Mechanistic studies applying in vivo methods (middle panel) and in vitro or ex vivo methods (right panel) can complement and expand the understanding of drug biotransformation by the human gut microbiome, including activation, inactivation, conversion into toxic metabolites and bioavailability. FMT, faecal microbiota transplantation.