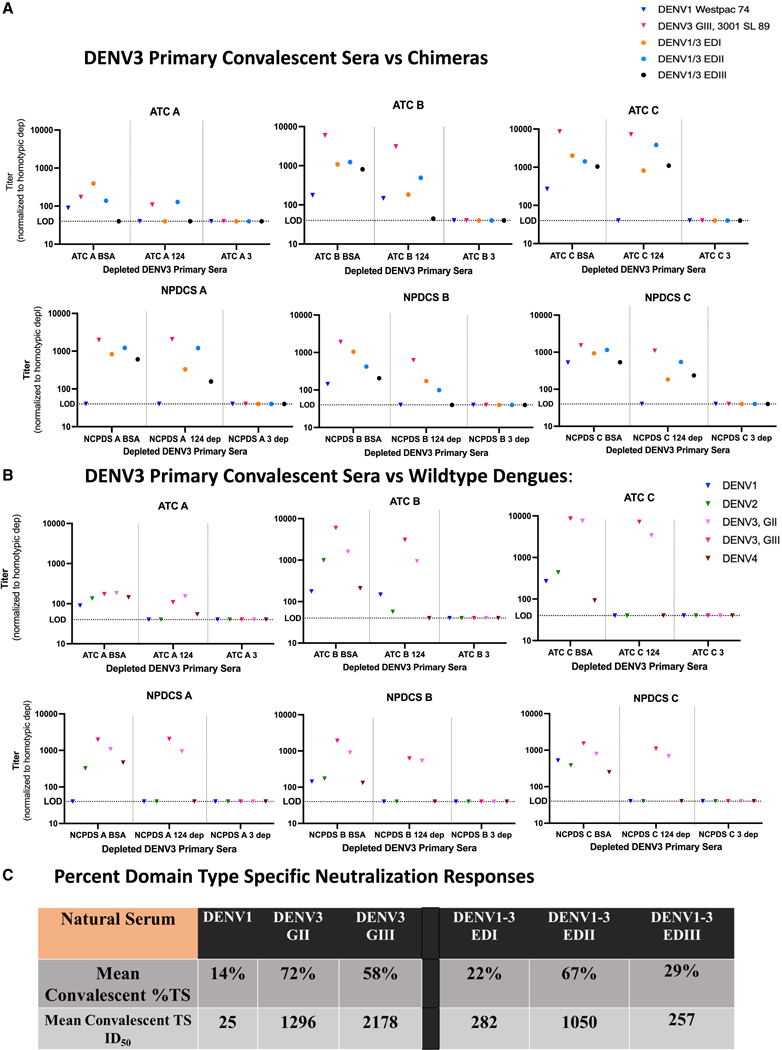
Figure 5. Primary DENV3 TS serum neutralization responses primarily target EDII after infection.
(A) ED-targeting of late convalescent primary infection sera. Six DENV3 primary natural serum samples, 3 from the adult Arbovirus Travelers Cohort (ATC A–C) and three from the Nicaraguan Pediatric Dengue Cohort Study (NPDCS A–C) were depleted with bovine serum albumin (BSA), DENV1, 2, and 4, or DENV3 virus on a magnetic bead, and the remaining sera tested for neutralization with the DENV1/3 EDI-EDIII panel.
(B) Wild-type and genotype outcomes. Late convalescent sera were used to neutralize wild-type viruses (DENV1, 2, 4, and DENV3 genotypes G-II and G-III). The BSA control represents total neutralization, the middle column (DENV1, 2, 4 depletion) represents the DENV3 TS nAb response, and the DENV3 depleted column represents any background neutralization which was then subtracted from the other columns.
(C) ED specific neutralization phenotypes. The mean DENV3 %TS antibody responses and mean DENV3 TS ID50 titers were calculated from the BSA and 1, 2, 4 depleted fractions to each virus across the late convalescent sera and represent the mean percentage or ID50 titer to each virus that is DENV3 TS compared with the control depletions, which contain both TS and CR antibody fractions.