Extended Data Fig. 4. Comparison to additional GPCR structures.
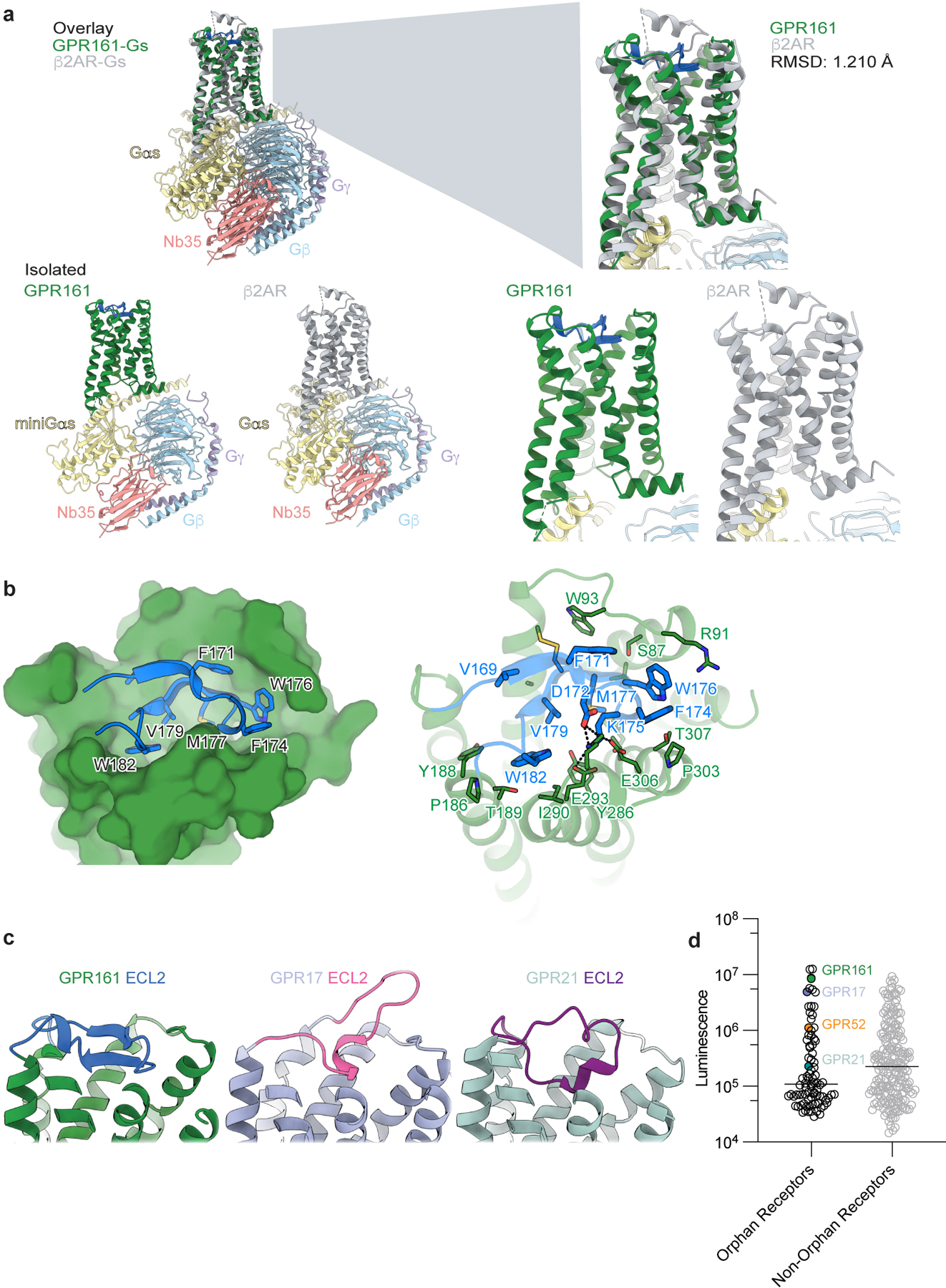
a) Structural comparison of GPR161 heterotrimer complex and β2AR heterotrimer complex (PDB ID: 3SN628). GPR161 has the same hallmarks of GPCR activation as the prototypical receptor, β2AR. b) View of the GPR161 ECL2 inside the canonical Class A GPCR binding site. ECL2 makes multiple hydrophobic interactions deep within the pocket. The superficial part of the pocket harbors ionic interactions between ECL2 and the binding pocket. c) Structural comparison of GPR161 to other orphan GPCRs with self-activating ECL2, including GPR17 (PDB ID: 7Y89) and GPR21 (PDB ID: 8HMV)31,32. The cis-interaction of ECL2 with the canonical ligand-binding site is seen across self-activating orphan GPCRs but the precise loop conformation changes between receptors. c) Luminescence for β-arrestin recruitment in the PRESTO-Tango assay when compared across 314 GPCRs (data replotted from Kroeze WM et al 201522, n = 4 for each target, shown as mean ± s.e.m. of technical replicates). GPR21 yields a signal slightly above the median. GPR52 yields a signal one order of magnitude above the median. GPR161 and GPR17 yield a signal about two orders of magnitude above the median.
