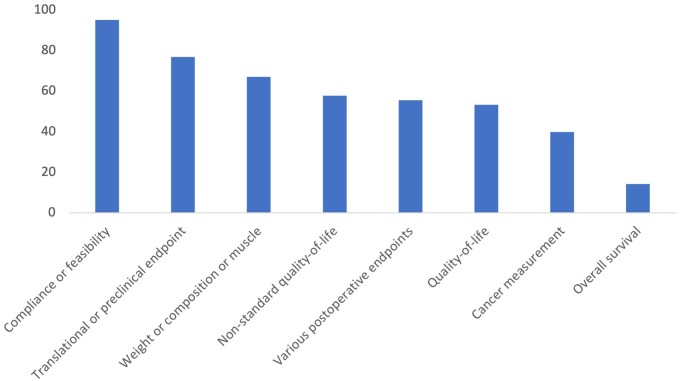
Figure 3.
Percentage of RCTs with achieved primary endpoint by endpoint categorization. Endpoints from RCTs testing dietary interventions in cancer patients were categorized into the following categories: Compliance or feasibility, Translational or preclinical, measurements of Weight or composition or muscle mass, Nonstandard quality-of-life, Various postoperative endpoints, Quality-of-life, Cancer measurements, and Overall survival. Quality-of-life endpoints were defined as validated measurements such as the EORTC QLQ-C30, FACIT-F, FACIT-G, and SF-36 health questionnaires. Nonstandard quality-of-life were measurements of symptom burden without the use of standardized scales, such as chemotherapy or radiation-induced adverse events, infection rates, unspecified measurement scales of QoL, and functional outcomes (such as fatigue). Cancer measurement endpoints were defined as response rates, clinical/biochemical recurrences, progression-free survival, relapse-free survival, and time until cancer recurrence.