Fig. 5. Validation of MD-124 efficacy in an ex-vivo skin infection model (A, B, C) and in-vivo systemic infection model in mice (D, E, F).
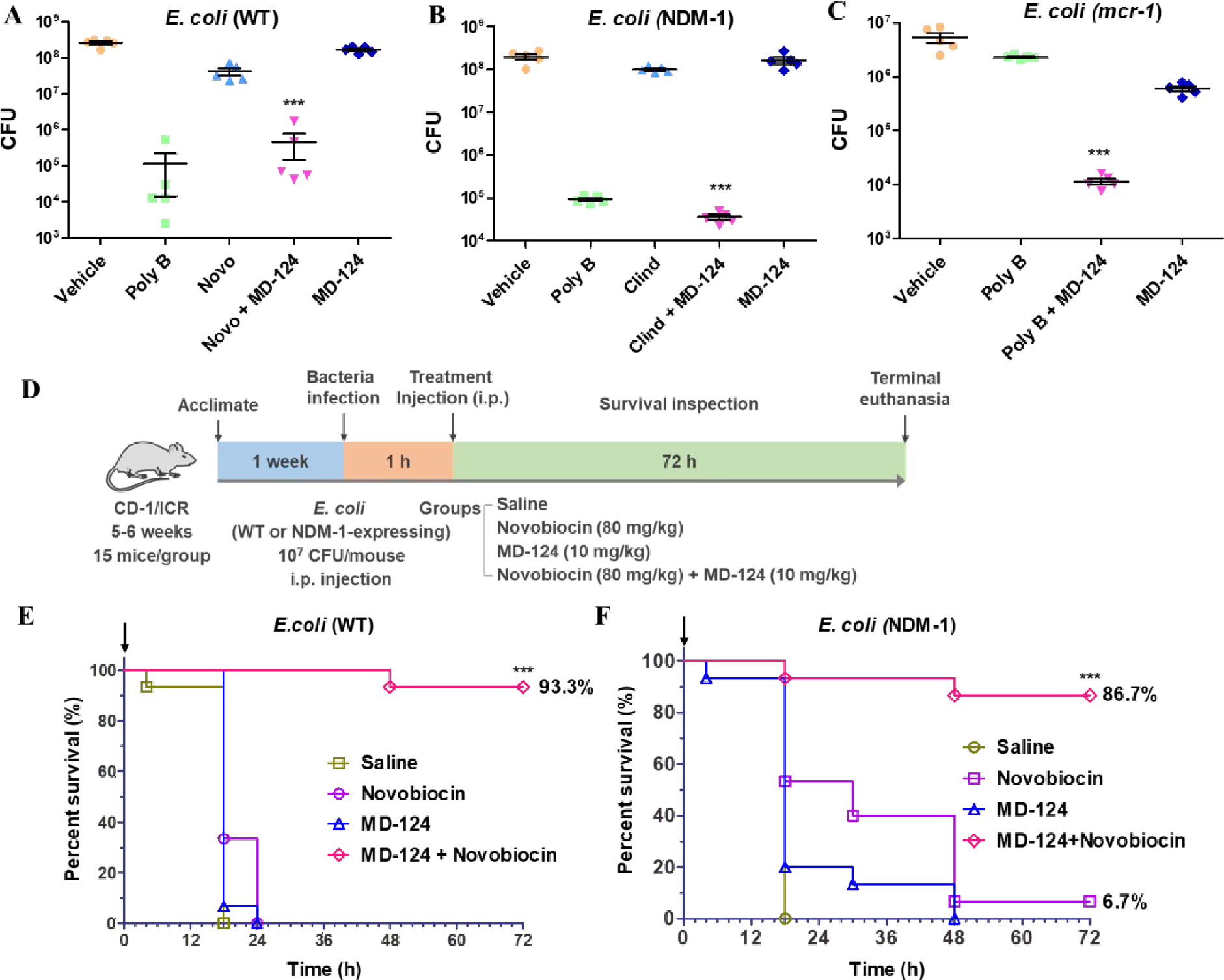
(A) Combination of MD-124 and novobiocin inhibited wild-type (WT) E. coli growth. Concentration (w/w) for polymyxin B (poly B), novobiocin (Novo) and MD-124 were 1‰, 4‰ and 1.5‰. The same concentration was used for novobiocin and MD-124 in the combination treatment groups (Novo + MD-124). (B) Combination of MD-124 and clindamycin inhibited the growth of NDM-1-expressing E. coli. Concentration (w/w) for polymyxin B (poly B), clindamycin (Clind) and MD-124 were 1‰, 3‰ and 1.5‰. The same concentration was used for clindamycin and MD-124 in the combination groups (Clind + MD-124). (C) Combination of MD-124 and polymyxin B inhibited the growth of mcr-1-expressing E. coli. Concentration (w/w) for polymyxin B (poly B) and MD-124 were 3‰ and 1.5‰. The same concentration was used for polymyxin B and MD-124 in the combination groups (Poly B + MD-124). For Fig. A, B, C, values are means ± SEM. n = 5, P values were determined using unpaired two-tailed Student’s t-tests. ***P < 0.001 vs antibiotics or MD-124 alone group. (D) Schematic illustration of the experimental procedures of a systemic infection model in mice. (E and F) MD-124 and novobiocin combinations significantly increased the survival rates of mice after infection by WT E. coli (E) and NDM-1-expressing E. coli (F). Arrows in Fig. E and F indicate the treatment time. The concentration for MD-124 and novobiocin is 10 mg/kg and 80 mg/kg respectively. The same concentration was used for novobiocin and MD-124 in the combination treatment groups. For Fig. E and F, n = 15 biologically independent animals per group. ***P < 0.001 vs antibiotics or MD-124 alone group. Statistical analysis using Log-rank (Mantel-Cox) test.
