Figure 7.
Venetoclax boosts mitochondrial metabolism via NF-κB to facilitate IS formation in NK cells
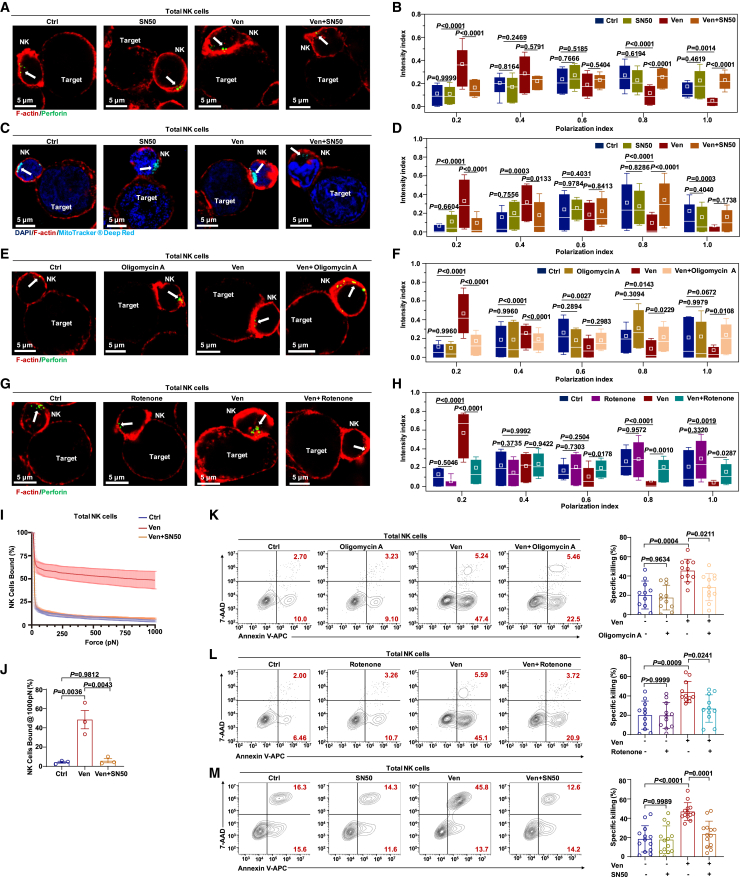
(A) Representative confocal images of cell conjugates after NK cell/KG-1a cell contact under the indicated conditions stained with F-actin (red) and perforin (green). NK cells (1 × 105 cells per well) treated with venetoclax (400 nM), SN50 (25 μg/mL), or venetoclax plus SN50 or left untreated were co-cultured with KG-1a cells at a 1:1 ratio for 1 h. White arrows indicate perforin.
(B) Granule-to-synapse distance quantified for 33–47 conjugates per group. The results represent three independent experiments. The distance from the perforin to the IS was determined as described in Figure S3G.
(C) MitoTracker Deep Red (cyan), F-actin (red), and DAPI (blue) staining in cell conjugates after NK cell/KG-1a cell contact under the indicated conditions. NK cells (1 × 105 cells per well) treated with venetoclax (400 nM), SN50 (25 μg/mL), or venetoclax plus SN50 or left untreated were co-cultured with KG-1a cells for 30 min at a ratio of 1:1. White arrows indicate mitochondria.
(D) Mitochondria-to-synapse distance quantified for 39–71 conjugates per group. The results represent three independent experiments. The distance from the mitochondria to the IS was determined as described in Figure S3G.
(E) F-actin (red) and perforin (green) staining in cell conjugates after NK cell/KG-1a cell contact under the indicated conditions. NK cells (1 × 105 cells per well) treated with venetoclax (400 nM), oligomycin A (200 nM), or venetoclax plus oligomycin A or left untreated were co-cultured with KG-1a cells for 1 h at a ratio of 1:1. White arrows indicate perforin.
(F) The granule-to-synapse distance quantified for 32–45 conjugates per group. The results represent three independent experiments. The distance from perforin to the IS was determined as described in Figure S3G.
(G) F-actin (red) and perforin (green) staining in cell conjugates after NK cell/KG-1a cell contact under the indicated conditions. NK cells (1 × 105 cells per well) treated with venetoclax (400 nM), rotenone (200 nM), or venetoclax plus rotenone or left untreated were co-cultured with KG-1a cells for 1 h at a ratio of 1:1. White arrows indicate perforin.
(H) The granule-to-synapse distance quantified for 24–63 conjugates per group. The results represent three independent experiments. The distance from perforin to the IS was determined as described in Figure S3G.
(I) The immune-synapse-binding avidity of NK cells, treated with venetoclax (400 nM) or venetoclax plus SN50 (25 μg/mL) or left untreated, to KG-1a cells was assessed via acoustic force microfluidic microscopy.
(J) Cell-binding avidity from (J) at 1,000 pN, n = 3.
(K and L) Representative flow cytometry plots and quantification of the specific killing ability of CB-NK cells (5 × 104 cells per well) treated with venetoclax (400 nM), oligomycin A (200 nM), rotenone (200 nM), venetoclax plus oligomycin A, or venetoclax plus rotenone or left untreated against KG-1a cells at a 2.5:1 ratio for 4 h (n = 11, biological replicates). The results represent six independent experiments. Representative flow plots showing NK cells derived from the same donor.
(M) Flow cytometry analysis of the percentage of Annexin V+ KG-1a cells co-cultured for 4 h at a 2.5:1 ratio with total NK cells (5 × 104 cells per well) pretreated with venetoclax (400 nM), SN50 (25 μg/mL), or venetoclax plus SN50 or left untreated (n = 13, biological replicates). The results represent six independent experiments.
Statistical significance was determined by one-way ANOVA with Tukey’s multiple comparisons test (B, D, F, H, and J–M). Data are presented as mean ± SD.
See also Figure S6.