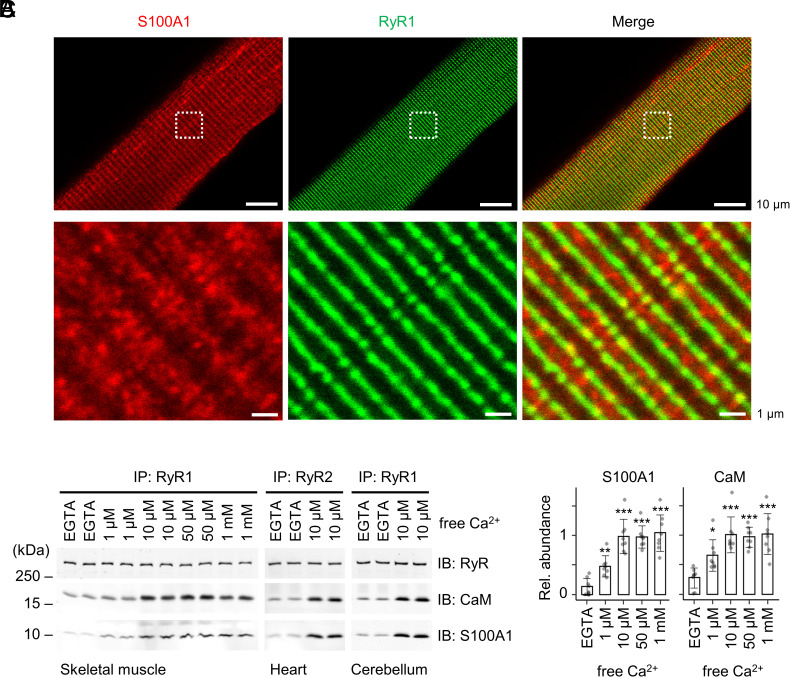
Fig. 6.
Interactions between endogenous S100A1 and RyR channels. (A) Confocal coimmunofluorescence images of S100A1 (red) and RyR2 (green) in skeletal myofibers (green) isolated from mouse EDL muscles. RyR1 exhibited a cross-striated pattern typical for the junctional sarcoplasmic reticulum and partially overlapping with S100A1. Dashed boxes indicate magnification shown below. Scale bar, 10 µm (Top) and 1 µm (Bottom). (B) Ca2+ dependency of coimmunoprecipitations (IP) between endogenous RyR channels and S100A1 versus CaM. RyR complexes were immunoprecipitated from skeletal muscle, heart muscle, and cerebellar lysates at indicated free [Ca2+] followed by immunoblotting against RyR, S100A1, and CaM. (C) Quantification of bound S100A1 and CaM in the IPs of RyR1 from the skeletal muscle. The relative abundance of S100A1/RyR1 (Left) and CaM/RyR1 (Right) is plotted against free [Ca2+] (mean ± SD; n = 8 per group) showing a tendentiously stronger Ca2+ dependence of the endogenous S100A1–RyR1 interaction compared to CaM. Two-tailed Student’s t test; *P < 0.05, **P < 0.01, ***P < 0.001.