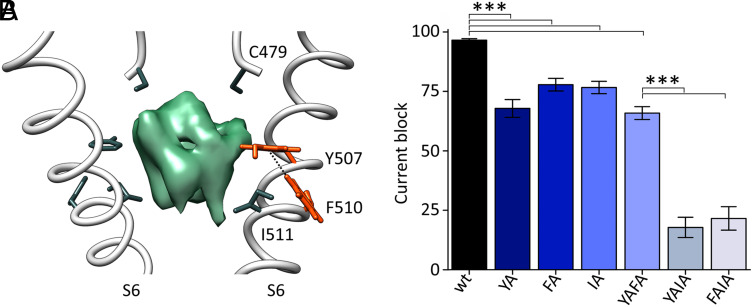
Fig. 4.
Role of F510 in ivabradine block. (A) Cross-membrane view of the HCN4 pore (S6) bound to ivabradine (PDB: 8OFI, SI Appendix, Table S1). For clarity, only two opposite subunits of the tetramer are shown. Residues directly or indirectly involved in ivabradine (green density) binding are shown as sticks and labeled: C479 from the SF and Y507 and I511 from S6 (RbHCN4 numbering). F510 develops a π-interaction (edge-to-face, 5.8 Å) with Y507. Side chains of both residues are shown as orange sticks, and their hydrogen atoms are shown. (B) Mean maximal block by 300 µM ivabradine of HCN4 wt and Y507A (YA), I511A (IA), Y507A-I511A (YAIA), Y507A-F510A (YAFA), and F510A-I511A (FAIA) mutant channels: 97.8 ± 0.6% (wt); 68.7 ± 3.8% (YA); 77.6 ± 2.6% (IA); 18.0 ± 4.3% (YAIA); 67.7 ± 2.2% (YAFA); 21.8 ± 5.0% (FAIA). Values are mean of n ≥ 3 experiments ± SEM. Statistical analysis was performed with one-way ANOVA, followed by Fisher’s test (***P < 0.001). The percentage of current block of YA, IA, and YAFA mutants is not statistically different, as well as the one of YAIA and FAIA.