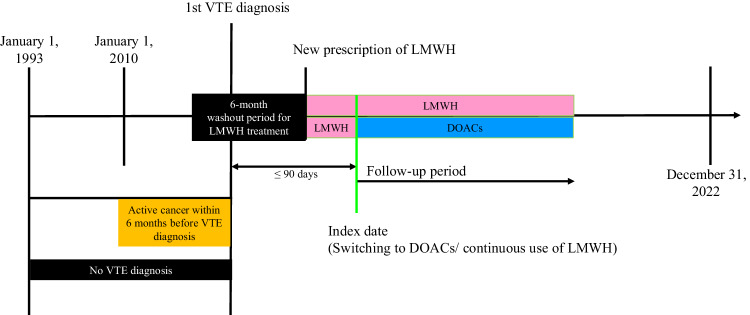
Fig. 3. Design of cohort study.
Patients with cancer-associated venous thrombosis (CAT) were identified as those with active cancer followed by an incident diagnosis of venous thromboembolism (VTE) between January 1, 2010, and December 31, 2022. Active cancer (yellow band) was defined as a new cancer diagnosis, recurrent cancer diagnosis, presence of metastasis, recent cancer-related treatment, or palliative care within the six months prior to the first VTE diagnosis. Switchers were defined as patients who switched from receiving low-molecular-weight heparin (LMWH, pink band) treatment to direct oral anticoagulants (DOACs, blue band). Non-switchers were patients who consistently received LMWH treatment (pink band). The start of initial LMWH treatment was determined as the date of the first recorded LMWH prescription following the 1st VTE diagnosis. Only patients who received LMWH treatment within 90 days after the CAT diagnosis were included in this study. Patients with a history of LMWH treatment within six months prior to the initial LMWH treatment were excluded as LMWH-experienced. For the switcher group, the index date was defined as the first date of DOAC treatment. A pseudo-index date was randomly assigned based on the distribution (mean ± standard deviation [SD]) of the time difference between the commencement of LMWH treatment and the switch to DOACs in the switcher group. Patients were followed up from the index date (green line) for six months or until the earliest occurrence of the outcome, death, or the end of the study period (December 31, 2022).