Abstract
Introduction:
Risk stratification tools to identify patients with diabetes and prediabetes at highest risk for heart failure (HF) are needed to inform cost-effective allocation of preventive therapies. Whether a biomarker score can meaningfully stratify HF risk is unknown.
Methods:
Participants free of cardiovascular disease from 3 cohort studies (ARIC, DHS, and MESA) were included. An integer-based biomarker score included high-sensitivity cTn-T≥6 ng/L, NT-proBNP≥125 pg/mL, hs-CRP≥3 mg/L, and left ventricular hypertrophy by electrocardiogram, with 1 point for each abnormal parameter. The 5-year risk of HF was estimated among participants with diabetes and prediabetes across biomarker score groups (0-4).
Results:
The primary analysis included 6,799 participants with dysglycemia (diabetes=33.2%, prediabetes=66.8%). The biomarker score demonstrated good discrimination and calibration for predicting 5-year and 10-year HF risk among prediabetes and well as diabetes cohorts. The 5-year risk of HF among individuals with a biomarker score of ≤1 was low and comparable to participants with euglycemia (0.78%). The 5-year risk for HF increased in a graded fashion with increasing biomarker score with the highest risk noted among those with score ≧ 3 (diabetes=12.0%; prediabetes=7.8%). The estimated number of HF events that could be prevented with use of an SGLT-2 inhibitor per 1,000 treated individuals over 5 years was 11 for all individuals with diabetes and ranged from 4 in the biomarker score = 0 group to 44 in the biomarker score ≥ 3 group.
Conclusions:
Among adults with diabetes and prediabetes, a biomarker score can stratify HF risk and inform allocation of HF prevention therapies.
Keywords: Biomarkers, Diabetes, Prediabetes, Risk Prediction, SGLT-2 Inhibitors
INTRODUCTION
Diabetes affects over 47 million adults in North America and is a well-established risk factor for heart failure (HF).(1,2) Although the incidence of diabetes has plateaued in the US, rates of prediabetes continues to grow.(1,3) Dysglycemia below diagnostic thresholds for diabetes has also been associated with chronic myocardial injury and abnormal cardiac structure and function(4,5) and higher risk of HF.(6) Thus, patients with diabetes and prediabetes are important high-risk populations for effective and efficient HF prevention strategies.
Sodium-glucose cotransporter-2 inhibitors (SGLT-2i) reduce the risk of HF among patients with prevalent diabetes.(7) Multi-specialty consensus recommendations and decision pathways recommend SGLT-2i for select high-risk patients, particularly those with established atherosclerotic cardiovascular disease (ASCVD).(8,9) However, there is little guidance for prescription of these therapies for those without established ASCVD, and even among patients with diabetes with clear indication for an SGLT-2i, prescription rates remain low.(10) Given most (~75%) patients with diabetes do not have established ASCVD, and the added cost of SGLT2i, effective risk stratification tools that accurately identify patients with diabetes at the highest risk for HF may help allocate SGLT-2i to those patients who are expected to derive the greatest absolute benefit. Moreover, such tools may help to also identify patients with prediabetes who may benefit from HF prevention strategies.
While conventional HF risk prediction tools have largely relied on traditional cardiovascular risk factors, there has been growing interest in incorporating blood- and electrocardiogram-based biomarkers to improve risk prediction, particularly among individuals without established cardiovascular disease (CVD).(11-14) Specifically, elevated levels of routinely collected biomarkers of cardiac injury, stress, and systemic inflammation, as well as left ventricular hypertrophy (LVH) are associated with higher risk of HF among healthy community dwelling adults.(13,15-17) Whether multiple biomarkers collectively can meaningfully stratify risk of incident HF in a high-risk population of patients with diabetes and prediabetes has not, to the best of our knowledge, been examined previously. Accordingly, we evaluated the application of a biomarker-based risk score in adults with diabetes and prediabetes to identify those at highest risk for incident HF, which could be used to inform the most cost-efficient use of effective preventive therapies, like SGLT-2i.
METHODS
Study population
Individual participant data were pooled from 3 epidemiologic cohort studies: Atherosclerosis Risk in Communities (ARIC) study, Dallas Heart Study (DHS), and Multiethnic Study of Atherosclerosis (MESA). The design, testing protocol, and HF event adjudication procedures of each study have been described previously and detailed in the supplemental methods.(18-20) Participants with a prevalent coronary heart disease, stroke, or HF and those with missing data on biomarkers interest or traditional cardiovascular risk factors were excluded. All participants provided written informed consent for study participation. The coordinating centers for each of the cohort studies approved the present study.
Clinical variables & assessment of diabetes and prediabetes
Baseline covariates were assessed among participants from all 3 cohorts in standardized examinations (Supplemental Methods).(18-20) Diabetes was defined using established clinical criteria, in each of the 3 cohorts.(18-20) In ARIC (visit 2,1990-92) and DHS (visit 1,2000-2002), diabetes was defined as: 1) self-reported provider diagnosis; 2) self-reported antihyperglycemic medication use; or 3) fasting plasma glucose (FPG)≥126 mg/dL; or 3) non-fasting plasma glucose ≥20 0 mg/dL.(21)’(22) In MESA (visit 1), diabetes was defined according to either self-reported use of antihyperglycemic medication or FPG ≥ 126 mg/dL.(23) Prediabetes was defined by FPG of 100 to <126mg/dL among participants without diabetes. Glycated hemoglobin levels were not available in DHS and MESA at their baseline visits and thus, were not used to define glycemic status.
Biomarker measurements
We a priori selected 4 cardiac and inflammatory biomarkers to include in the composite biomarker score based on prior associations of each with incident HF.(13, 15-17) These included high-sensitivity cardiac troponin-T (hs-cTnT), N-terminal pro-B-type natriuretic peptide (NT-proBNP), high-sensitivity C-reactive protein (hs-CRP), and electrocardiogram-based LVH (ECG-LVH, Sokolow-Lyon criteria) (detailed protocols for their measurement in Supplemental Methods. Thresholds used to define elevated levels of hs-cTnT, NT-proBNP, and hs-CRP were selected based on clinical practice limits of quantification and previously established cut-points as follows: hs-cTnT≥6 ng/L, NT-proBNP≥125 pg/mL, and hs-CRP≥3 mg/L.(13,14,24)
Outcome of interest: Incident heart failure
The primary outcome was incident HF. The HF adjudication protocols for each study are detailed in the Supplemental Methods.
Statistical analysis
Participant-level data across all 3 cohorts were pooled. Baseline characteristics were compared among participants with diabetes versus prediabetes using chi-square test for categorical variables and Kruskal-Wallis test for continuous variables. In the overall study cohort, multivariable Cox proportional hazard models were constructed to evaluate the independent association of biomarkers of interest (hs-cTnT, NT-proBNP, hs-CRP, ECG-LVH) with the risk of incident HF. Separate models were constructed using categorical and continuous measures of the biomarkers for the overall cohort and across subgroups with diabetes and prediabetes. Models were constructed with serial adjustments for biomarkers of interest alone (model-1) and demographics (age, sex, race), CV risk factors (smoking status, BMI, systolic BP, diabetes/prediabetes status, total cholesterol, HDL-c, estimated glomerular filtration rate [GFR], FPG), medication use (statin, anti-hypertensive medication), and study cohort (model-2).
The biomarker score incorporated each of the 4 biomarkers (hs-cTnT[+], NT-proBNP[+], hs-CRP[+], and LVH by ECG[+]) with 1-point for each abnormal biomarker, with a maximal score of 4. Baseline characteristics and risk of HF were assessed across 4 biomarker score groups: very low (score:0), low (score:1), intermediate (score:2), and high (score:3-4).
The association of the biomarker score with risk of HF was evaluated using the same models described above. The discrimination and calibration performance of the biomarker score alone in predicting 5-year and 10-year risk of HF were assessed and compared with a clinical risk factor-based model that included covariates described in model 2 above. Harrell concordance index (C-index) was calculated for assessment of model discrimination and differences in C-indices between the biomarker score and clinical risk factor model was evaluated using bootstrapping. The Grønnesby and Borgan test was used to assess calibration for the adjusted models.
The 5- and 10-year risk of incident HF was calculated for participants with diabetes and prediabetes across the biomarker score groups using Kaplan-Meier estimates. The HF risk among euglycemic individuals in the pooled cohort was included as a reference. Number of HF events that could be prevented per 1,000 participants treated with an SGLT-2i for 5- and 10-years was calculated across biomarker score groups and prespecified subgroups (age>65 years, 10-year ASCVD risk>10%) using previously reported relative risk reduction estimate of 36% for SGLT-2i.(7) Number-needed-to-screen to prevent 1 HF event over 5- and 10-years was calculated for each biomarker score group by dividing the corresponding number-needed-to-treat by the prevalence of that score in the study population with diabetes. Sensitivity analyses were performed to evaluate the 5- and 10-year risk of incident HF across key subgroups stratified by BMI (< and ≥ 30 kg/m2) and eGFR (< and ≥ 60 mL/min/1.73m2).
All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., NC, USA).
RESULTS
The pooled cohorts included 17,360 participants (ARIC-51.2%, DHS-14.2%, MESA-34.6%) (e-Figure-1). Among the 6,799 participants with dysglycemia (women:50.2%, Black: 28.8%), 33.2% had diabetes and 66.8% had prediabetes. Compared with those with prediabetes, participants with diabetes were older, had higher burden of cardiovascular risk factors, higher hs-cTnT and hs-CRP levels, and higher prevalence of ECG-LVH (e-Table-1). During median follow-up of 17 years, 891 participants (13.1%) with dysglycemia developed HF [diabetes: 19.9%(n=448); prediabetes: 9.8%(n=443)]. Among participants with euglycemia, 5.5% developed HF during follow-up.
Predictors of HF risk among participants with diabetes or prediabetes
Among individuals with dysglycemia, older age, male sex, prevalent diabetes (versus prediabetes), smoking, higher systolic BP, BMI, FPG, and total cholesterol were each independently associated with higher risk of HF (e-Table-2). Among biomarkers, elevated levels of hs-cTnT, NT-proBNP, hs-CRP, and presence of ECG-LVH were each independently associated with higher risk of HF in both unadjusted and adjusted models (Table-1). The overall pattern of association of these biomarkers with risk of HF was largely comparable in subgroups of individuals with diabetes and prediabetes. (e-Table-3).
Table 1. Multivariable adjusted association of biomarkers and risk of HF among participants with diabetes or prediabetes.
| Diabetes or prediabetes | ||||
|---|---|---|---|---|
| Model 1 | Model 2 | |||
| aHR (95% CI) | P value | aHR (95% CI) | P value | |
| Biomarker categories | ||||
| hs-cTnT (+) | 2.37 (2.08, 2.71) | <0.001 | 1.56 (1.35, 1.81) | <0.001 |
| NT-proBNP (+) | 2.46 (2.10, 2.88) | <0.001 | 2.07 (1.75, 2.46) | <0.001 |
| hs-CRP (+) | 1.64 (1.44, 1.87) | <0.001 | 1.28 (1.11, 1.48) | <0.001 |
| LVH by ECG (+) | 1.53 (1.28, 1.83) | <0.001 | 1.34 (1.11, 1.62) | 0.002 |
| Continuous biomarker levels | ||||
| Log hs-cTnT per 1 SD increase | 1.60 (1.51, 1.70) | <0.001 | 1.30 (1.21, 1.40) | <0.001 |
| Log NT-proBNP per 1 SD increase | 1.58 (1.47, 1.69) | <0.001 | 1.56 (1.44, 1.69) | <0.001 |
| Log hs-CRP per 1 SD increase | 1.32 (1.24, 1.41) | <0.001 | 1.16 (1.07, 1.25) | <0.001 |
| Voltage by ECG per 1 SD increase | 1.11 (1.04, 1.18) | 0.002 | 1.10 (1.02, 1.18) | 0.009 |
Hs-cTnT (+): hs-cTnT ≥ 6 ng/L; NT-proBNP (+): NT-proBNP ≥ 125 pg/mL; hs-CRP (+): hs-CRP ≥ 3 mg/L; LVH by ECG (+) was determined according to Sokolow-Lyon criteria.
Model 1 includes the following covariates: biomarker categories (hs-cTnT [+], NT-proBNP [+], hs-CRP [+], and LVH by ECG [+]) or continuous levels (log hs-cTnT, log NT-proBNP, log hs-CRP, voltage by ECG).
Model 2 includes the following covariates: demographics (age, sex, race), traditional CV risk factors (smoking status, BMI, systolic BP, prediabetes / diabetes status, total cholesterol, HDL cholesterol, estimated GFR, FPG), medication use (statin, anti-hypertensive medication), study cohort, biomarker categories (hs-cTnT [+], NT-proBNP [+], hs-CRP [+], and LVH by ECG [+]) or continuous levels (log hs-cTnT, log NT-proBNP, log hs-CRP, voltage by ECG).
Abbreviations: BMI = body mass index; BP = blood pressure; CV = cardiovascular; ECG = electrocardiogram; FPG = fasting plasma glucose; GFR = glomerular filtration rate; HDL = high-density lipoprotein; hs-CRP = high-sensitivity C-reactive protein; hs-cTnT = high-sensitivity cardiac troponin T; LVH = left ventricular hypertrophy; NT-proBNP = N-terminal pro-B-type natriuretic peptide; SD = standard deviation.
Association of the biomarker score with risk of HF
Distribution of the biomarker score and baseline characteristics of participants with diabetes and prediabetes across biomarker score groups are shown in e-Table-4. Among participants with diabetes, 66.6% had very low to low biomarker scores (score ≤1), while 25.1% had an intermediate (score=2) and 8.3% had a high biomarker score (score ≥ 3). A similar distribution pattern of biomarker scores was seen among participants with prediabetes, where 79.7%, 16.2%, and 4.1% had a very low to low (score≤1), intermediate (score=2), and high biomarker score (score≥3), respectively. Individuals with higher biomarker scores were older, more commonly Black, and had higher systolic BP, BMI, and estimated risk of ASCVD.
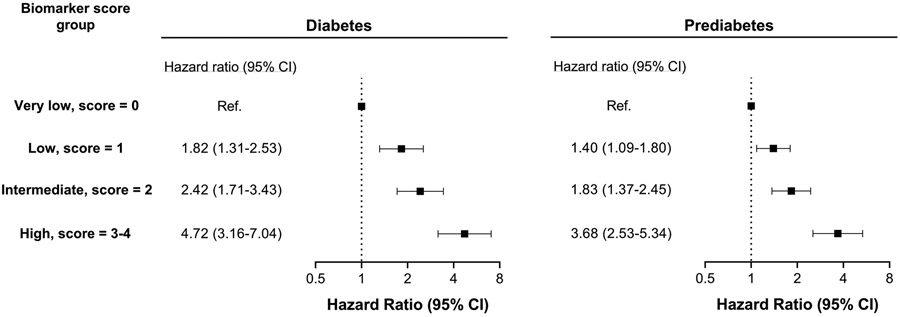
The cumulative incidence of HF increased in a graded fashion across increasing biomarker score groups for individuals with diabetes as well as prediabetes (e-Figure-2). In multivariable-adjusted Cox analysis, a significant graded association was observed between biomarker score groups and risk of HF among participants with diabetes and prediabetes independent of other potential confounders, with the highest risk noted among individuals in the high score group (score≥3) (Figure-1).
Figure 1. Multivariable adjusted association of biomarker score groups with risk of HF among participants with diabetes and prediabetes.
Covariates included demographics (age, sex, race), cardiovascular risk factors (smoking status, body mass index, systolic blood pressure, total cholesterol, high-density lipoprotein cholesterol, estimated glomerular filtration rate, fasting plasma glucose), medication use (statin, anti-hypertensive medication), study cohort.
5-year and 10-year HF risk across biomarker score groups
The biomarker score demonstrated good discrimination and calibration for predicting 5- and 10-year risk of HF in dysglycemia (e-Table-5). The discrimination performance of the biomarker score alone was comparable to the clinical risk factor model for predicting 5- and 10-year risk of HF in diabetes and predicting 5-year risk of HF in prediabetes. For 10-year HF risk in prediabetes, the discrimination performance of the biomarker score was modestly lower than that of the clinical risk factors model. Calibration was good for each of the HF risk prediction models among participants with diabetes or prediabetes (p>0.05 for all).
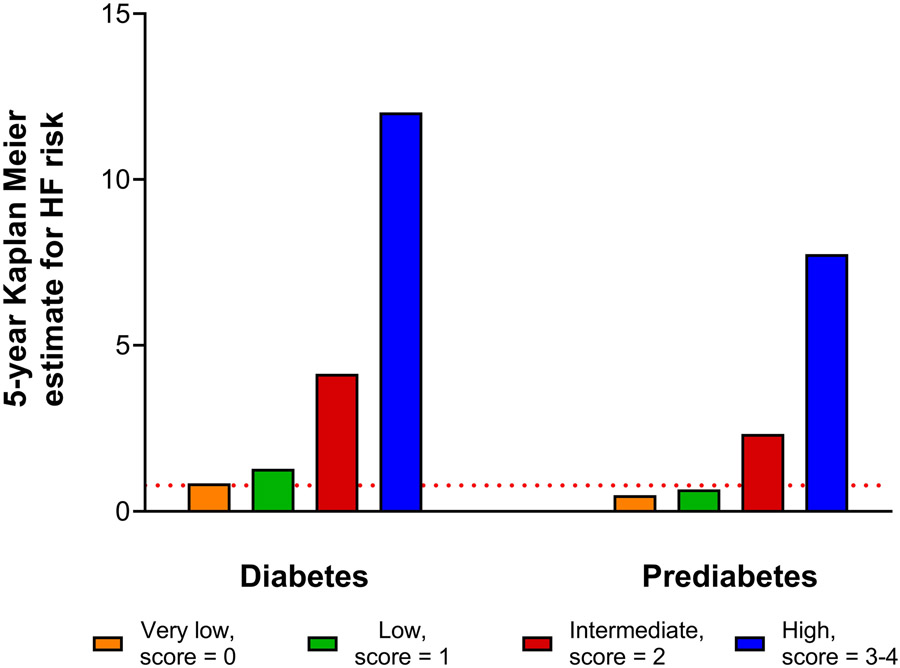
The 5-year risk of HF among participants with diabetes and prediabetes with a biomarker score of 0 or 1 was very low (diabetes: 0.85% to 1.29%; prediabetes: 0.49% to 0.67%) and comparable to that observed among individuals with euglycemia (0.78%). The 5-year risk of HF increased in a graded fashion across increasing biomarker score categories such that individuals with diabetes and prediabetes in the high biomarker score group (≥3) had 15- and 10-fold higher risk of HF (vs. individuals with euglycemia), respectively (Figure-2). Similarly, a similar pattern of graded increase was noted for 10-year HF risk estimates across increasing biomarker score groups for individuals with diabetes and prediabetes. (e-Figure-3). The graded 5- and 10-year risk of HF was similar in subgroup analyses stratified by BMI and eGFR categories (e-Figure-4 and 5).
Figure 2. 5-year Kaplan Meier estimates for heart failure risk among participants with diabetes and prediabetes stratified by biomarker score group.
The red dotted line represents the 5-year Kaplan Meier estimate for heart failure in participants with euglycemia (0.78%).
Among participants with diabetes, individuals with biomarker score of ≥ 3 comprised 8.3% of the population and accounted for 35.5% and 28.4% of all HF events within 5- and 10-years, respectively (Table-2). Similarly, among participants with prediabetes, individuals with biomarker score of ≥3 comprised 4.1% of the population and accounted for 26.9% and 22.4% of all HF events within 5- and 10-years, respectively (e-Table-6).
Table 2. Subgroup analysis of HF events and number of HF events prevented per 1,000 participants with diabetes treated with an SGLT-2 inhibitor versus placebo among participants with diabetes.
Based on a pooled analysis of SGLT-2 inhibitor cardiovascular outcome trials, a 36% relative risk reduction in HF was used to calculate the absolute risk reduction at 5- and 10-years.(7)
| Total no. of participants |
Proportion of all participants with diabetes |
Total no. of HF events (%) | No. of HF events prevented / 1,000 participants treated with an SGLT-2 inhibitor |
|||
|---|---|---|---|---|---|---|
| Over 5-year follow-up |
Over 10-year follow up |
For 5 years | For 10 years | |||
| All participants with diabetes | 2,256 | 100% | 62 (100%) | 176 (100%) | 11 | 30 |
| Participants with diabetes > 65 years of age | 473 | 21.0% | 25 (40.3%) | 56 (31.8%) | 20 | 47 |
| Participants with diabetes and PCE-estimated 10-year ASCVD risk >10% | 1,432 | 63.5% | 53 (85.5%) | 143 (81.3%) | 14 | 39 |
| Participants with diabetes and a biomarker score ≥2 | 754 | 33.4% | 45 (72.6%) | 109 (61.9%) | 22 | 56 |
| Participants with diabetes and a biomarker score ≥3 | 188 | 8.3% | 22 (35.5%) | 50 (28.4%) | 44 | 103 |
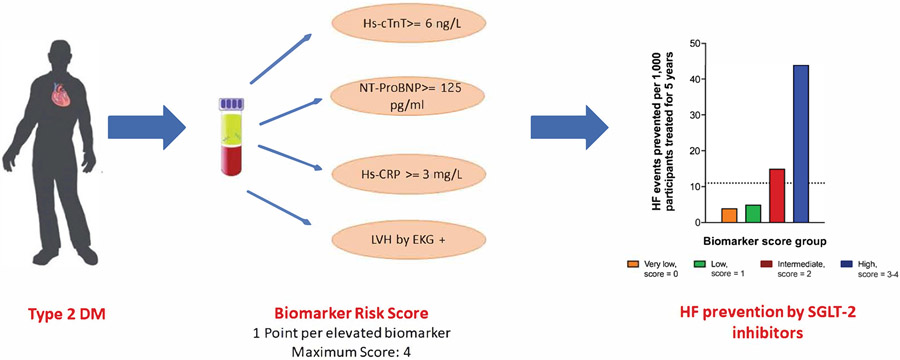
For every 1,000 participants with diabetes treated with an SGLT-2i, the estimated number of HF events that could be prevented was 11 over 5 years and 30 over 10 years. When stratified by the biomarker score, the number of HF events that could be prevented by SGLT-2i increased across increasing score groups. In the high biomarker score group, SGLT-2i could prevent 44 HF events over 5 years (vs. 4 or 5 HF events prevented in the 0 or 1 score groups, respectively) and 103 HF events over 10 years (vs. 12 or 20 HF events prevented in the 0 or 1 score groups, respectively) per 1,000 treated participants. (Central Illustration, e-Figure-6). The number-needed-to-screen to prevent 1 HF event over 5-years was 268 and 278 in the intermediate (score=2) and high biomarker groups (score=3-4), respectively (e-Figure-7). The number-needed-to-screen to prevent 1 HF event over 10 years are shown in e-Figure-8.
Central Illustration: Biomarker risk score to identify patient with diabetes who are a high risk of heart failure and may benefit from SGLT-2 inhibitors.
The black dotted line represents the number of HF events prevented per 1,000 participants with diabetes, irrespective of biomarker score group, treated with an SGLT-2 inhibitor for 5 years (11 HF events). Based on a pooled analysis of SGLT-2 inhibitor cardiovascular outcome trials, a 36% relative risk reduction in HF was used to calculate the absolute risk reduction at 5-years.(7) Abbreviations: HF = heart failure; SGLT-2 = sodium-glucose cotransporter-2.
DISCUSSION
Among participants with dysglycemia without CVD, biomarkers of chronic myocardial injury, neurohormonal stress, systemic inflammation, and LVH were independently associated with higher risk of HF. Furthermore, a simple integer score based on the levels of these biomarkers demonstrated good discrimination, calibration, and risk stratification for predicting HF risk. Individuals with biomarker scores ≤1 had very low risk of HF comparable with that observed among adults with euglycemia. In contrast, the risk of HF was considerably higher among individuals with high biomarker scores (score ≥3). Participants with diabetes with a high biomarker score represented <10% of the cohort but accounted for 35% of HF events over 5-years. A biomarker score ≥3 identified individuals with diabetes who were most likely to benefit from SGLT-2i for prevention of HF (Central Illustration). Taken together, these findings underscore the potential utility of a biomarker-based approach for HF risk stratification and allocation of HF prevention therapies among patients with dysglycemia.
Patients with diabetes, even those who achieve target levels of conventional risk factors, including HbA1c<7%, face high HF risks.(25) Risk of HF increases across the glycemic spectrum, even below diagnostic thresholds for diabetes.(26-28) Prediabetes is associated with myocardial injury, subclinical cardiac dysfunction (stage B HF), and development of clinical HF.(5,6,29,30) In contrast with prior studies,(31) prediabetes was associated with higher risk of incident HF. This may be related to differences in study populations, including age (older vs. younger) and heterogeneity in risk across groups stratified by biomarkers. Additionally, the long 17-year median follow-up in the present study may have been necessary to identify HF risk. Up to 10% of patients with prediabetes progress to diabetes annually and this transition is associated with higher risk of CVD, including HF.(32,33) Thus, prediabetes represents an early stage of dysglycemia that could be targeted for prevention of HF screened using cardiac and inflammatory biomarkers.(6,23,34) We extend these observations by incorporating biomarkers into a comprehensive and pragmatic risk score.
Several strategies for predicting HF risk among patients with diabetes have been developed. Previous studies created simple integer-based risk scores using readily available clinical variables to stratify risk of HF.(11,12) However, these risk scores were developed among clinical trial populations that included patients who had diabetes and prevalent CVD such as ASCVD and HF. Furthermore, cardiac and inflammatory biomarkers were not included in these risk scores. We build upon these prior studies by evaluating community-dwelling adults without CVD and examined several biomarkers with established prognostic importance. Additionally, we examined individuals with prediabetes who represent a large and expanding proportion of the population with dysglycemia and are important targets for implementation of HF prevention strategies.(1,6,29,30) Currently, there is limited guidance regarding HF risk assessment in patients with prediabetes .(9) Our biomarker score identified a 16-fold gradient of 5-year HF risk across increasing scores in prediabetes. A high biomarker score was present in 4% of individuals with prediabetes and accounted for 27% of all HF events over 5 years. Thus, high biomarker score could identify such high-risk individuals with prediabetes who may benefit from more aggressive management strategies for HF prevention including intensive BP control and aggressive lifestyle modification.(35)
Incorporation of biomarkers for risk stratification can also guide implementation of strategies for prevention of HF in patients with diabetes. Despite their demonstrated efficacy of SGLT-2i,(9) therapeutic uptake has been slow,(10,36) likely in part related to financial barriers. A more cost-efficient approach may help target preventive interventions to individuals with diabetes at highest risk for HF. A high biomarker score was noted in <10% of all individuals with diabetes but accounted for 35% of all HF events over 5-year follow-up. These individuals identified with high biomarker scores would be expected to derive significant benefits from a more aggressive approach to HF prevention that may include initiation of effective but relatively costly therapies such as SGLT-2i. We observed that SGLT-2 therapies would prevent 44 HF events over 5-years per 1,000 treated individuals with diabetes and high biomarker score. In contrast, treatment of all participants with diabetes would prevent only 11 HF events per 1,000 treated individuals. Future studies are needed to determine if a biomarker score-guided approach to allocation of novel, effective pharmacotherapies for diabetes may be cost effective. Furthermore, while financial costs of these medications are expected to decline over time, targeting these therapies to the highest risk individuals should lead to the largest absolute risk reductions. Additionally, a biomarker-based approach to risk stratification may help inform integration of these effective therapies into clinical practice. Glucagon-like peptide-1 receptor agonists (GLP-1RA) are a separate antihyperglycemic class of medications that have also demonstrated cardioprotective benefits, particularly reductions in ASCVD events.(37) Further studies are needed to identify individuals with diabetes who are at enriched risk for ASCVD and may preferentially benefit from GLP-1RA.
Three-quarters of individuals with prediabetes and two-thirds of individuals with diabetes had a low biomarker score that was associated with 5-year risk of HF that was lower than or comparable with that for patients with euglycemia. The biomarker score-based approach to risk stratification can help guide the treatment discussions and shared-decision making process regarding implementation of potential HF preventive therapies among these low-risk individuals.
Several limitations to our study are noteworthy. First, the study population may not represent a contemporary cohort as these epidemiologic cohort studies began enrollment prior to the availability of diabetes therapies like SGLT-2i. Because no participants were taking SGLT-2i at the time of study enrollment, we were able to estimate the number of HF events that could be prevented with initiation of an SGLT-2i. Second, biomarkers were assessed at a single point in time and the contribution of changes in biomarker levels to risk of HF was not assessed. However, a single assessment of the biomarker score provides a simple, pragmatic approach to risk stratification. Third, the biomarker score did not incorporate continuous measures of the biomarkers. While categorical biomarkers may miss important information at the ends of the distribution, a categorical approach facilitates the ease of use and may be more readily adopted. Fourth, LVH was assessed by electrocardiogram rather than echocardiogram which was not consistently performed in each of the three cohorts. Electrocardiogram testing is widely available and may be a pragmatic approach to risk stratification. Data regarding measures of insulin resistance and other metabolic parameters were not consistently collected across pooled cohorts; more detailed phenotyping of the dysglycemic cohorts was thus not possible. Finally, HF event ascertainment differed across cohort studies and ARIC included HF events based on ICD codes which are susceptible to misclassification.(38,39).
In conclusion, among community-dwelling adults with diabetes and prediabetes without prior CVD, a simple biomarker-based integer risk score can inform HF risk stratification. Individuals with diabetes and prediabetes who had low biomarker scores had a low risk of incident HF similar to those with euglycemia. Among individuals with diabetes and high biomarker scores, the risk of HF was higher and prescription of SGLT-2i would be expected to prevent a greater number of incident HF events. Future studies are needed with measurement of these biomarkers to guide allocation of preventive therapies in clinical practice.
Supplementary Material
PERSPECTIVES.
Competency in medical knowledge:
A simple, integer, biomarker-based risk score can identify patients with diabetes and prediabetes who are at an increased risk for incident heart failure.
Translational outlook:
Future studies are needed to determine whether assessment of these biomarkers may guide allocation of therapies to prevent heart failure in patients with diabetes and prediabetes
ACKNOWLEDGEMENTS
The authors thank the participants, staff, and investigators of the ARIC, DHS, and MESA studies for their contributions.
STUDY FUNDING
This study was supported by the Texas Health Resources Clinical Scholarship to Dr. Pandey.
The ARIC study is conducted as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN2 68201100006C, HHSN268201100007C, HHSN268201100008C, HHSN26820 1100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201 100012C).
The Dallas Heart Study was funded by a grant from the Donald W. Reynolds Foundation. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001105 to the University of Texas Southwestern Medical Center.
A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The Multi-Ethnic Study of Atherosclerosis was supported by R01 HL071739 and contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, and N01HC 95169 from the National Heart, Lung, and Blood Institute. Reagents for the NT-proBNP and high sensitivity cardiac troponin T assays were donated by Roche Diagnostics.
DISCLOSURES
Dr. Vaduganathan is supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst (NIH/NCATS Award UL 1TR002541) and serves on advisory boards for Amgen, AstraZeneca, Baxter Healthcare, Bayer AG, Boehringer Ingelheim, Cytokinetics, and Relypsa. Dr. de Lemos reports grant support from Roche Diagnostics and Abbott Diagnostics, consulting fees from Roche Diagnosics, Abbott Diagnosics, Ortho Clinical Diagnostics, Siemen’s Health Care Diagnostics, Quidel Cardiovascular, Inc, Novo Nordisk, Amgen, Regeneron, Eli Lilly, and Esperion. Dr’s DeFilippi and de Lemos have been awarded a patent pending (US Patent Number: 15/309,754) entitled: “Methods for Assessing Differential Risk for Developing Heart Failure.” DKM has received personal fees for trial leadership from GlaxoSmithKline, Janssen, Lexicon, AstraZeneca, Sanofi Aventis, Boehringer Ingelheim, Merck & Co, Pfizer, Novo Nordisk, Eisai Inc., Esperion, Lilly USA, and personal consultancy fees from AstraZeneca, Lilly USA, Boehringer Ingelheim, Merck & Co, Novo Nordisk, Metavant, Applied Therapeutics, Sanofi Aventis, Afimmune. Dr. Everett reports personal consultancy fees from Amarin, Amgen, Gilead, FDA, Merck & Co., NIDDK, and Roche Diagnostics outside the present work. Dr. Everett reports significant investigator-initiated grant funding from the NHLBI outside the present work.
ABBRIVIATIONS
- HF
Heart failure
- SGLT-2i
Sodium-glucose cotransporter-2 inhibitors
- ASCVD
Atherosclerotic cardiovascular disease
- LVH
Left ventricular hypertrophy
- ARIC
Atherosclerosis Risk in Communities study
- DHS
Dallas Heart Study
- MESA
Multiethnic Study of Atherosclerosis
- BP
Blood pressure
- HDL-C
high-density lipoprotein cholesterol
- hs-cTnT
high-sensitivity cardiac troponin-T
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
- hs-CRP
high-sensitivity C-reactive protein
- FPG
Fasting plasma glucose
Footnotes
Presentation: Lifestyle and Cardiometabolic Health Early Career Investigator Award Session at American Heart Association Scientific Sessions 2020
REFERENCES
- 1.National Diabetes Statistics Report. https://www.cdc.gov/diabetes/data/statistics/statistics-report.html. Accessed May 13, 2020.
- 2.Khan H, Anker SD, Januzzi JL Jr. et al. Heart Failure Epidemiology in Patients With Diabetes Mellitus Without Coronary Heart Disease. J Card Fail 2019;25:78–86. [DOI] [PubMed] [Google Scholar]
- 3.Bullard KM, Saydah SH, Imperatore G et al. Secular changes in U.S. Prediabetes prevalence defined by hemoglobin A1c and fasting plasma glucose: National Health and Nutrition Examination Surveys, 1999-2010. Diabetes Care 2013;36:2286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubin J, Matsushita K, Ballantyne CM, Hoogeveen R, Coresh J, Selvin E. Chronic hyperglycemia and subclinical myocardial injury. J Am Coll Cardiol 2012;59:484–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skali H, Shah A, Gupta DK et al. Cardiac structure and function across the glycemic spectrum in elderly men and women free of prevalent heart disease: the Atherosclerosis Risk In the Community study. Circ Heart Fail 2015;8:448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selvin E, Lazo M, Chen Y et al. Diabetes mellitus, prediabetes, and incidence of subclinical myocardial damage. Circulation 2014;130:1374–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zelniker TA, Wiviott SD, Raz I et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019;393:31–39. [DOI] [PubMed] [Google Scholar]
- 8.Das SR, Everett BM, Birtcher KK et al. 2018 ACC Expert Consensus Decision Pathway on Novel Therapies for Cardiovascular Risk Reduction in Patients With Type 2 Diabetes and Atherosclerotic Cardiovascular Disease: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol 2018;72:3200–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Diabetes Association. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020;43:S111–S134. [DOI] [PubMed] [Google Scholar]
- 10.Arnold SV, Inzucchi SE, Tang F et al. Real-world use and modeled impact of glucose-lowering therapies evaluated in recent cardiovascular outcomes trials: An NCDR(R) Research to Practice project. Eur J Prev Cardiol 2017;24:1637–1645. [DOI] [PubMed] [Google Scholar]
- 11.Berg DD, Wiviott SD, Scirica BM et al. Heart Failure Risk Stratification and Efficacy of Sodium-Glucose Cotransporter-2 Inhibitors in Patients With Type 2 Diabetes Mellitus. Circulation 2019;140:1569–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segar MW, Vaduganathan M, Patel KV et al. Machine Learning to Predict the Risk of Incident Heart Failure Hospitalization Among Patients With Diabetes: The WATCH-DM Risk Score. Diabetes Care 2019;42:2298–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Lemos JA, Ayers CR, Levine BD et al. Multimodality Strategy for Cardiovascular Risk Assessment: Performance in 2 Population-Based Cohorts. Circulation 2017;135:2119–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandey A, Patel KV, Vongpatanasin W et al. Incorporation of Biomarkers Into Risk Assessment for Allocation of Antihypertensive Medication According to the 2017 ACC/AHA High Blood Pressure Guideline: A Pooled Cohort Analysis. Circulation 2019;140:2076–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang TJ, Larson MG, Levy D et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med 2004;350:655–63. [DOI] [PubMed] [Google Scholar]
- 16.Kalogeropoulos A, Georgiopoulou V, Psaty BM et al. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol 2010;55:2129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pandey A, Keshvani N, Ayers C et al. Association of Cardiac Injury and Malignant Left Ventricular Hypertrophy With Risk of Heart Failure in African Americans: The Jackson Heart Study. JAMA Cardiol 2019;4:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 19.Victor RG, Haley RW, Willett DL et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol 2004;93:1473–80. [DOI] [PubMed] [Google Scholar]
- 20.Bild DE, Bluemke DA, Burke GL et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- 21.Schneider AL, Kalyani RR, Golden S et al. Diabetes and Prediabetes and Risk of Hospitalization: The ARIC Study. Diabetes Care 2016;39:772–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neeland IJ, Turer AT, Ayers CR et al. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA 2012;308:1150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen K, Fan W, Bertoni A et al. N-terminal Pro B-type Natriuretic Peptide and High-sensitivity Cardiac Troponin as Markers for Heart Failure and Cardiovascular Disease Risks According to Glucose Status (from MESA). Am J Cardiol 2020;125:1194–1201. [DOI] [PubMed] [Google Scholar]
- 24.Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol. 2013. Oct 8;62(15):1365–72. [DOI] [PubMed] [Google Scholar]
- 25.Rawshani A, Rawshani A, Franzen S et al. Risk Factors, Mortality, and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med 2018;379:633–644. [DOI] [PubMed] [Google Scholar]
- 26.Iribarren C, Karter AJ, Go AS et al. Glycemic control and heart failure among adult patients with diabetes. Circulation 2001;103:2668–73. [DOI] [PubMed] [Google Scholar]
- 27.Segar MW, Patel KV, Vaduganathan M et al. Association of Long-term Change and Variability in Glycemia With Risk of Incident Heart Failure Among Patients With Type 2 Diabetes: A Secondary Analysis of the ACCORD Trial. Diabetes Care 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsushita K, Blecker S, Pazin-Filho A et al. The association of hemoglobin a1c with incident heart failure among people without diabetes: the atherosclerosis risk in communities study. Diabetes 2010;59:2020–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Sarnola K, Ruotsalainen S et al. The metabolic syndrome predicts incident congestive heart failure: a 20-year follow-up study of elderly Finns. Atherosclerosis 2010;210:237–42. [DOI] [PubMed] [Google Scholar]
- 30.Spahillari A, Talegawkar S, Correa A et al. Ideal Cardiovascular Health, Cardiovascular Remodeling, and Heart Failure in Blacks: The Jackson Heart Study. Circ Heart Fail 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deedwania P, Patel K, Fonarow GC et al. Prediabetes is not an independent risk factor for incident heart failure, other cardiovascular events or mortality in older adults: findings from a population-based cohort study. Int J Cardiol 2013;168:3616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimaki M. Prediabetes: a high-risk state for diabetes development. Lancet 2012;379:2279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rijkelijkhuizen JM, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD, Dekker JM. High risk of cardiovascular mortality in individuals with impaired fasting glucose is explained by conversion to diabetes: the Hoorn study. Diabetes Care 2007;30:332–6. [DOI] [PubMed] [Google Scholar]
- 34.Gori M, Gupta DK, Claggett B et al. Natriuretic Peptide and High-Sensitivity Troponin for Cardiovascular Risk Prediction in Diabetes: The ARIC Study. Diabetes Care 2016;39:677–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bress AP, King JB, Kreider KE et al. Effect of Intensive Versus Standard Blood Pressure Treatment According to Baseline Prediabetes Status: A Post Hoc Analysis of a Randomized Trial. Diabetes Care 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arnold SV, de Lemos JA, Rosenson RS et al. Use of Guideline-Recommended Risk Reduction Strategies Among Patients With Diabetes and Atherosclerotic Cardiovascular Disease. Circulation 2019;140:618–620. [DOI] [PubMed] [Google Scholar]
- 37.Kristensen SL, Rorth R, Jhund PS et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol 2019;7:776–785. [DOI] [PubMed] [Google Scholar]
- 38.Rosamond WD, Chang PP, Baggett C et al. Classification of heart failure in the ARIC study: a comparison of diagnostic criteria. Circ Heart Fail 2012;5:152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rector TS, Wickstrom SL, Shah M et al. Specificity and sensitivity of claims-based algorithms for identifying members of Medicare+Choice health plans that have chronic medical conditions. Health Serv Res 2004;39:1839–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.