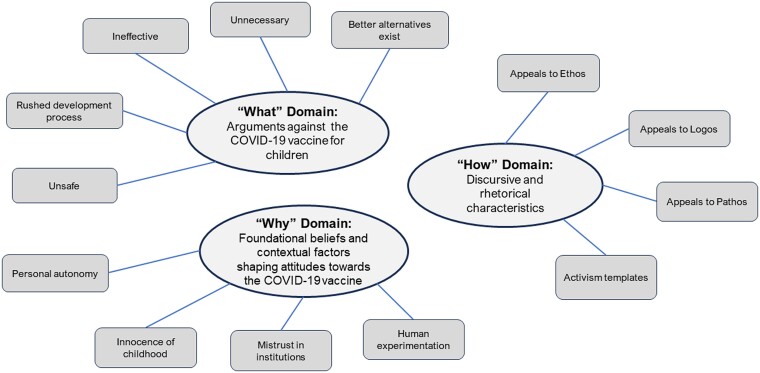
Figure 1.
Schematic of domains and themes emerging from analysis of public comments on the EUA of COVID-19 vaccines for pediatric use. Source: Authors’ own analysis of 149 897 public comments submitted to Docket FDA-2021-N-1088 from October 13 to 25, 2021, and Docket FDA-2022-N-0082 from February 4 to 14, 2022. The dockets pertained to amendment of the EUA for the Pfizer-BioNTech COVID-19 mRNA vaccines for administration to children aged 5-11 years and 6 months to 4 years, respectively. Notes: N/A.