Abstract
The focus of aging research has shifted from increasing lifespan to enhancing healthspan to reduce the time spent living with disability. Despite significant efforts to develop biomarkers of aging, few studies have focused on biomarkers of healthspan. We developed a proteomics-based signature of healthspan (healthspan proteomic score (HPS)) using data from the UK Biobank Pharma Proteomics Project (53,018 individuals and 2920 proteins). A lower HPS was associated with higher mortality risk and several age-related conditions, such as COPD, diabetes, heart failure, cancer, myocardial infarction, dementia, and stroke. HPS showed superior predictive accuracy for these outcomes compared to chronological age and biological age measures. Proteins associated with HPS were enriched in hallmark pathways such as immune response, inflammation, cellular signaling, and metabolic regulation. Our findings demonstrate the validity of HPS, making it a valuable tool for assessing healthspan and as a potential surrogate marker in geroscience-guided studies.
Over recent decades, global life expectancy has significantly increased, especially in developing countries. However, the corresponding healthy life expectancy, defined as years lived in full health since birth, has failed to keep up the pace1. The gap between healthspan and lifespan highlights a substantial challenge posed by chronic diseases among older adults2, emphasizing the need for novel approaches to narrow the gap and prolong disease-free longevity3. The geroscience approach is a potential solution, addressing the hypothesis that targeting the hallmarks of aging, rather than individual diseases of aging, may prevent or delay the onset of multiple age-related conditions4.
Unlike lifespan, which has a universal definition, there is no consensus on the definition of healthspan5,6. Previous research has suggested characterizing healthy aging in five domains: physical capability, cognitive function, physiological and musculoskeletal, endocrine, and immune functions5,7. For practical purposes, healthspan typically refers to the period of life spent in good health, free from the chronic diseases and disabilities of aging6. Studies aiming to evaluate the effects of interventions on healthspan are challenging due to the need for long follow-up lengths and large sample sizes of healthy individuals to observe the outcomes of interest. Thus, developing surrogate biomarkers that can predict healthspan is crucial for improving the feasibility of clinical trials to test interventions to prolong healthspan and lifespan.
Composite biomarkers incorporating multiple measures are more robust in predicting age-related outcomes than single biomarkers8–10. Several composite biomarkers for predicting lifespan or mortality have been developed using clinical biomarkers11,12 or omics data11,13–15. However, to date, no composite biomarker measures have been developed based on a healthspan definition. To mitigate this gap, we developed a proteomics-based healthspan biomarker (healthspan proteomic score, HPS) using chronological age and expression data of 2,920 proteins at the UK Biobank (UKB) baseline/recruitment (2006–10). In line with previous studies16,17, we defined healthspan as the number of years from birth without a major chronic medical condition, including cancer (excluding non-melanoma skin cancer), diabetes (type I diabetes, type II diabetes, and malnutrition-related diabetes), heart failure, myocardial infarction (MI), stroke, chronic obstructive pulmonary disease (COPD), dementia, or death. Participants from the UKB-PPP were followed up during a mean follow-up of 13.5 years. Using an independent internal cohort, HPS was validated against chronological age and biological age measures using clinical biomarkers or proteins. In addition, we investigated the biological processes associated with healthspan through gene set enrichment analysis.
Results
UKB PPP participants free from conditions in the healthspan condition at baseline
Of 502,269 active UKB participants, 53,018 were included in the UKB Pharma Proteomics Project (PPP) and had proteomic data available, as released by the UKB. Of those included in the UKB PPP, 43,119 (81.3%) participants had not been diagnosed with any condition included in the healthspan definition at baseline (Supplementary Table 1). Their mean age was 56 years (SD=8.2, range 39 to 70), and the majority were of European descent (93.8%) and female (55.8%). 34.3% of participants held a college or university degree, and the mean level of material deprivation, as measured by the Townsend deprivation index, was lower than the UK population average (−1.3 vs. 0). Additionally, 43.4% were previous or current smokers, and the mean BMI was 27.1 (SD=4.6). The prevalence of hypertension and hypercholesterolemia was 23.2% and 11.2%, respectively (Supplementary Table 2).
Baseline characteristics of UKB PPP participants ending healthspan during follow-up
After a mean follow-up of 13.5 years (from baseline to death or last follow-up, whichever occurred first), 12,427 (28.8%) developed at least one condition in the healthspan definition, and the first diagnosis was at the mean age of 66.7 years (SD=8.1). After excluding participants with multiple first-diagnosed conditions in the healthspan definition (n=1,214, 9.8%), cancer was the most common first-diagnosed condition (n=4,882, 44%), followed by MI (n=2,036, 18.2%), diabetes (n=1,455, 13.0%), and then COPD (n=1,131, 10.1%). Compared to participants who developed any condition in the healthspan definition during follow-up, participants who remained healthy were younger and more likely to be female or non-white. They had better socioeconomic status, healthier lifestyles, and a lower prevalence of hypertension and hypercholesterolemia at baseline (Supplementary Table 2).
Healthspan Proteomic Score (HPS) development using UKB PPP participants free from conditions in the healthspan definition at baseline
Participants who did not have any conditions in the healthspan definition at baseline (n=43,119) were randomly split into training (70%, n=30,184) and test sets (30%, n=12,935), where 2,296 (7.6%) and 989 (7.6%) participants died during follow-up, respectively (Supplementary Figure 1). The training and test samples shared similar participant characteristics at baseline (Supplementary Table 3).
We developed the HPS using the training set data, including 2,920 proteins and chronological age. In the variable selection, we selected chronological age and 86 proteins based on a Least Absolute Shrinkage and Selection Operator (LASSO) penalized Cox regression model for achieving a more parsimonious model with a close to the optimal deviance for predicting healthspan, following the one-standard-error rule18 (lambda 0.009277) (Supplementary Table 4). Then, chronological age and the selected proteins were used to fit a Gompertz model (Supplementary Table 4), where we calculated the 10-year risk of reaching the end of healthspan (rh), which was used to derive the healthspan proteomic score (HPS) by 1-rh. The lower the HPS, the higher the risk of ending healthspan.
HPS and the number of healthspan conditions at baseline among UKB PPP participants
HPS followed a left-skewed distribution in UKB PPP participants free from the healthspan conditions (Figure 1A). The distribution shifted to the left and showed a right tail as the number of healthspan conditions increased. The median HPS in those who had developed one or more conditions in the healthspan definition (0.70 [1 condition], 0.49 [2 conditions], 0.36 [3 conditions], 0.17 [4 conditions], 0.07 [5 conditions]) was significantly lower than that (0.84) in those without any condition included in the healthspan definition at baseline (p<0.001). Among participants with a chronic medical condition at baseline (n=8,136), those with cancer showed the highest median HPS (0.78), and those with MI (0.63), diabetes (0.62), or heart failure (0.62) showed the lowest median HPS (Figure 1B).
Figure 1. Healthspan Proteomic Score (HPS) distributions in UKB PPP participants, separated by the number of healthspan conditions at baseline (Figure 1A), and within those with a specific healthspan condition only at baseline (Figure 1B).
HPS and participant characteristics at baseline in the test set
Among the participants in the test set without any condition in the healthspan definition at baseline, the median HPS was significantly lower in males (vs. females), older adults (≥60 years vs. <60 years), former or current smokers (vs. non-smokers), obese participants (BMI≥30 vs. BMI<30), and participants diagnosed with hypertension or hypercholesterolemia before or at baseline (p<0.001, Supplementary Figure 2).
HPS and chronological age, biological age measures, as well as aging traits at baseline in the test set
HPS showed moderately negative correlations with chronological age (Spearman correlation r= −0.73) and previous composite biological age measures (Supplementary Figure 3): proteomic aging clock (PAC) trained to predict mortality15 (r= −0.87), PhenoAge11 (r= −0.79) and BioAge19 (r= −0.74) to predict mortality and chronological age, respectively. Moreover, HPS had a low negative correlation with a 49-item frailty index20 (r= −0.21), based on a wide range of self-reported health deficits, and a low positive correlation with leukocyte telomere length21 (r= 0.21) (Supplementary Figure 3). In addition, HPS was negatively correlated with BMI (r= −32), systolic blood pressure (r= −0.37), and reaction time based on a cognitive function test (r= −0.26) (Supplementary Figure 3). HPS showed a positive correlation with usual walking pace (r= 0.23) and a minimal correlation with maximal grip strength (r= −0.01) (Supplementary Figure 3). After regressing out the effect of chronological age, HPS continued to show a moderate correlation with PAC proteomic age (r= −0.65) but had lower correlations with PhenoAge (r= −0.39) and BioAge (r= −0.10) (Supplementary Figure 4).
HPS at baseline and risks of ending healthspan, mortality, and age-related conditions during follow-up in the test set
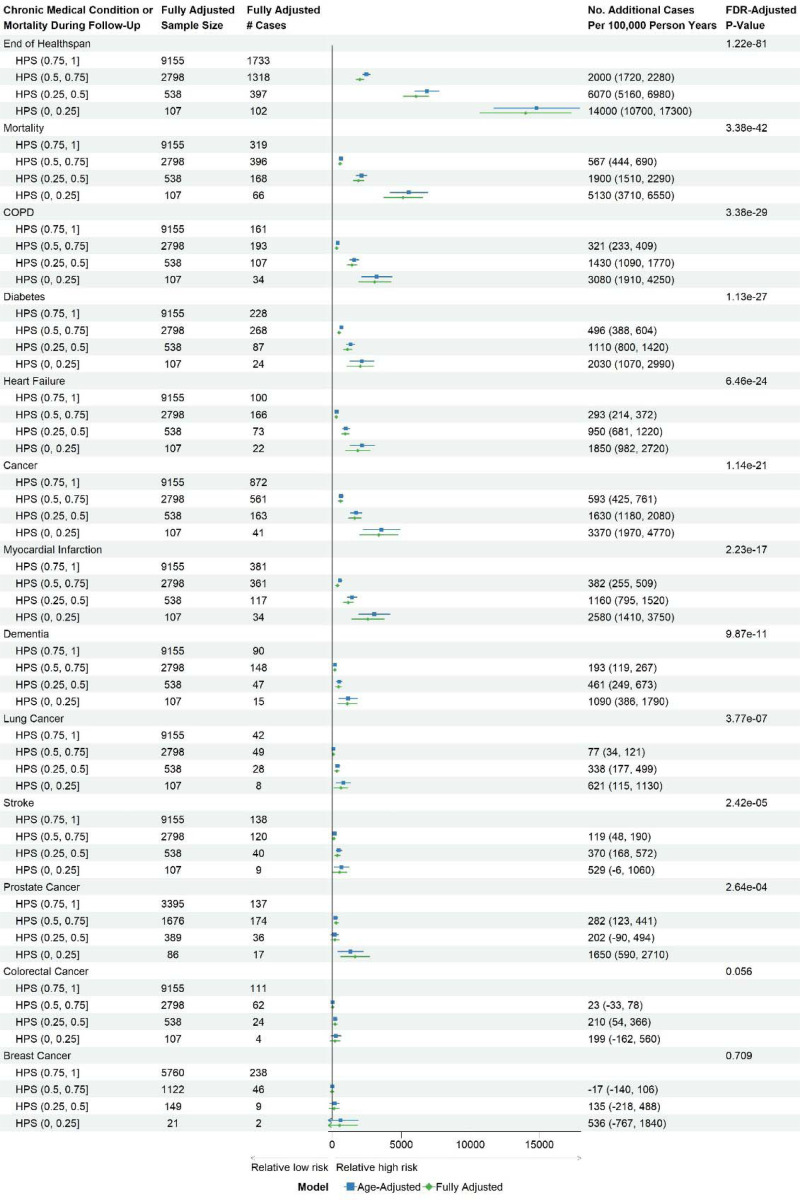
HPS was significantly associated with the time from baseline to the diagnosis of a first healthspan condition in the test set after adjusting for chronological age and other covariates at baseline (Supplementary Table 1): sex, ethnicity, education, Townsend deprivation index, BMI, smoking status, hypertension, hypercholesterolemia, and UKB PPP consortium selection for specific disease or ancestry interests (false discovery rate [FDR]-adjusted p-value, pFDR-adj=1.22×10−81). Using the HPS range of 0.75 to 1 as the reference (with the lowest risk of ending healthspan in the next 10 years among the four equal-interval groups of HPS), the risk of ending healthspan increased as HPS decreased, with an additional 2,000 cases per 100,000 person-years in participants with an HPS range from 0.5 to 0.75, 6,070 cases and 14,000 cases per 100,000 person-years in those with the ranges from 0.25 to 0.5 and from 0 to 0.25, respectively (Figure 2).
Figure 2. Risk differences comparing healthspan proteomic score (HPS) of (0.5, 0.75], (0.25, 0.5], or (0, 0.25) to HPS (0.75, 1] at baseline for ending healthspan or developing conditions in the healthspan definition using disease-free participants in the test set.
Fully adjusted covariates: age, sex, ethnicity, BMI, education, Townsend deprivation index, smoking status, hypertension, hypercholesterolemia, and UKB PPP consortium selection.
Lower HPS was significantly associated with conditions in the healthspan definition (pFDR-adj<0.05), particularly mortality, incident COPD, and diabetes (Figure 2). Among specific cancer diagnoses, a lower HPS was significantly associated with lung cancer and prostate cancer (pFDR-adj<0.05), but there was no significant association with colorectal cancer or breast cancer in females (pFDR-adj>0.05) (Figure 2). In addition, a lower HPS was associated with other medical conditions that were not included in the healthspan definition, such as pneumonia, chronic kidney disease, delirium, and osteoarthritis (pFDR-adj<0.05) (Supplementary Figure 5).
For sensitivity analysis, we dichotomized the HPS using the cut-off of 0.73 (“Low: HPS<0.73 vs. High: HPS≥0.73”). This threshold corresponded to the first quartile of HPS in the UKB PPP participants free from the healthspan conditions, effectively separating participants with and without healthspan conditions at baseline (Figure 1A). A low HPS score was associated with higher risks of conditions in the healthspan definition and other medical conditions (Supplementary Figures S6 and S7). Interestingly, the association between low HPS and healthspan was stronger in younger adults (<60 vs. ≥60 years), previous or current smokers (vs. never smokers), and obese participants (BMI≥30 vs. <30) (Supplementary Figure S8). Previous or current smokers also showed a stronger association with mortality (Supplementary Figure S9).
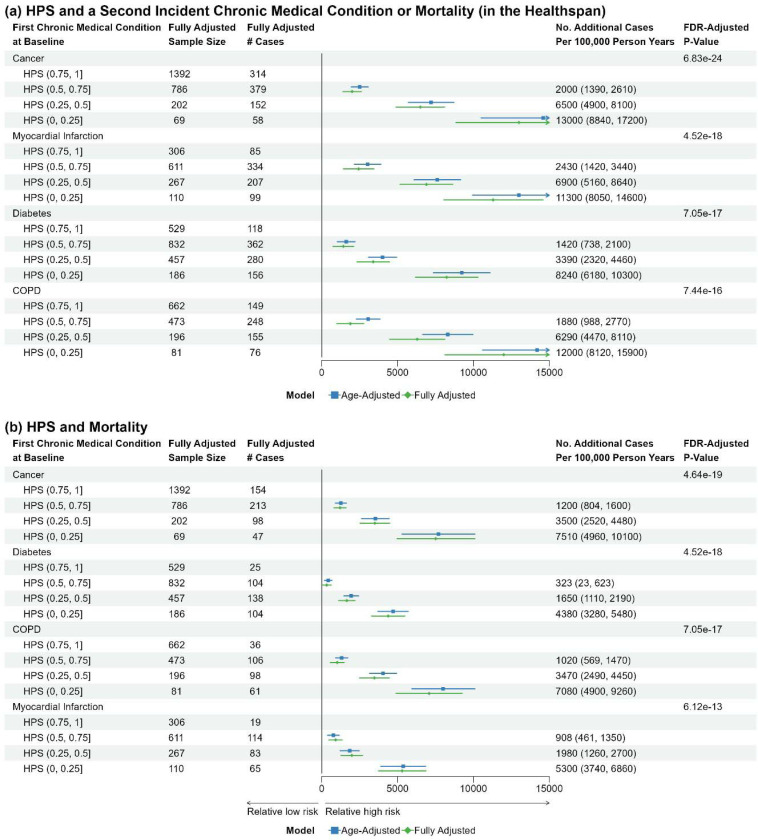
HPS at baseline and a second healthspan condition or mortality during follow-up among UKB PPP participants with a specific healthspan condition at baseline
We further investigated how HPS at baseline is associated with the development of a second healthspan condition and mortality during follow-up in those with a specific healthspan condition at baseline, considering the most prevalent healthspan conditions at baseline: cancer, MI, diabetes, and COPD. HPS was strongly associated with a second healthspan condition and mortality during follow-up, regardless of the pre-existing healthspan condition (Figure 3).
Figure 3. Risk differences comparing healthspan proteomic score (HPS) of (0.5, 0.75], (0.25, 0.5], or (0, 0.25) to HPS (0.75, 1] at baseline for a second healthspan condition (Figure 5a), or mortality (Figure 5b), in participants initially presenting with a healthspan condition at baseline.
Fully adjusted baseline covariates: age, sex, ethnicity, BMI, education, Townsend, smoking, hypertension, hypercholesterolemia, and UKB PPP consortium selection.
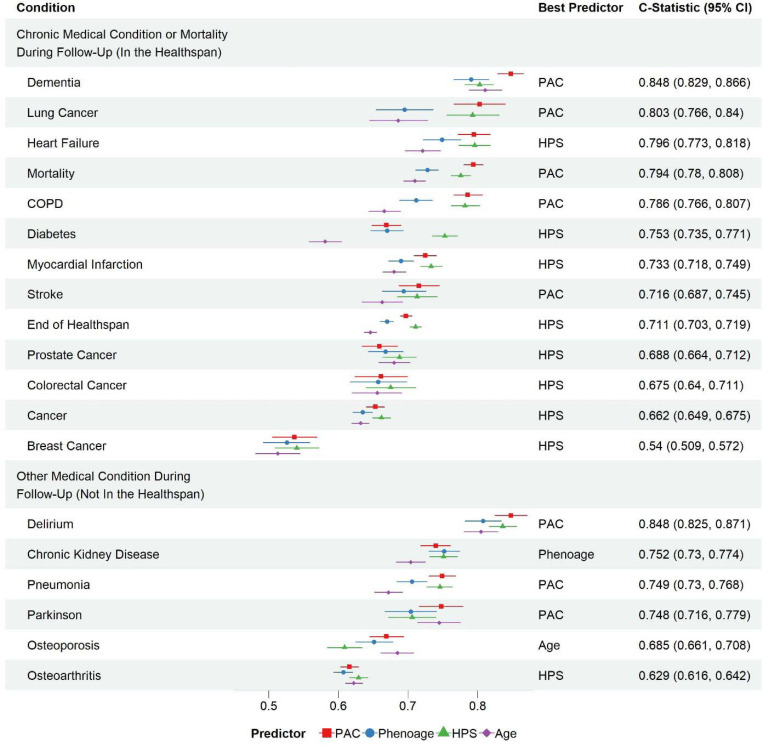
Joint analysis of HPS and PAC proteomic age groups at baseline and the risks of conditions in the healthspan definition and other medical conditions during follow-up in the test set
PAC is a recently developed proteomics-based clock to predict mortality15. Both HPS and PAC proteomic age strongly predicted conditions in the healthspan definition and other medical conditions (Figure 4). However, HPS was better at predicting diabetes, while PAC proteomic age was better at predicting dementia and osteoporosis. Compared to HPS and PAC proteomic age, PhenoAge and chronological age were less predictive across conditions, except chronic kidney disease and osteoporosis, which were best predicted by PhenoAge and chronological age, respectively (Figure 4).
Figure 4. Predictive power of healthspan proteomic score (HPS) versus proteomic aging clock (PAC) and PhenoAge assessed by C-statistics for time to the end of healthspan, incident diseases, and mortality using the test set data.
Despite their high predictive power for mortality and healthspan, HPS and PAC showed distinct predictive profiles for chronic diseases, suggesting that different biological processes underlie their measurements. We hypothesized that low HPS and high PAC significantly increased the risks of adverse health outcomes than low HPS or high PAC alone. To test the hypothesis, we compared the groups of HPS (high or low) and PAC (high or low) for the risks of ending healthspan, mortality, and medical conditions during follow-up. We categorized HPS and PAC at baseline using the cutoffs 0.73 and 58, respectively, corresponding to the first and third quartiles among UKB PPP participants. Either cutoff effectively separated participants with and without conditions included in the healthspan definition, as illustrated in Figure 1A and Supplementary Figure 10.
Among the participants in the test set with complete covariate data, 8,783 participants were in the high HPS and low PAC group (better biological health), who were compared with 704 in the low HPS and low PAC group (intermediate biological health), 762 in the high HPS and high PAC group (intermediate biological health), and 2,349 in the low HPS and high PAC group (worse biological health). The low HPS and high PAC showed significantly higher risk than the additive risk associated with low HPS alone and high PAC alone for mortality and end of healthspan (FDR-adjusted interaction p=1.20×10−5 [mortality], 0.002 [end of healthspan]) (Figure 5). Similar association patterns across the HPS and PAC groups were found with conditions in the healthspan definition such as heart failure, MI, stroke, and COPD (Figure 5), and other medical conditions, including pneumonia and chronic kidney disease (Supplementary Figure 11).
Figure 5. Risk differences comparing more adverse HPS and PAC groups to high HPS and low PAC group for mortality, end of healthspan, and incident chronic medical conditions in the healthspan definition in the test set.
Fully adjusted covariates: age, sex, ethnicity, BMI, education, Townsend, smoking, hypertension, hypercholesterolemia, UKB PPP consortium selection, and the conditions in the definition of healthspan.
Gene set enrichment analysis of proteins associated with HPS
Of 2,920 proteins analyzed, 1,398 showed significant associations (Bonferroni-corrected p<0.05) with HPS after adjusting for covariates at baseline (age, sex, ethnicity, education, Townsend deprivation index, BMI, smoking status, hypertension, and hypercholesterolemia, and UKB PPP consortium selection) (Supplementary Table 5). Those significantly upregulated or downregulated proteins in the low HPS group are highlighted in Figure 6A (mean difference between the low and high HPS group after inverse normal transformation greater than 0.4 SD or smaller than 0.4 SD, chosen to label a maximal number of genes without losing clarity). The significant proteins (Bonferroni-corrected p<0.05) showed enrichment in 26 hallmark gene sets, including immune response, inflammation, cellular signaling, and metabolic regulation (Figure 6B).
Figure 6. HPS-associated proteins and enriched hallmark gene sets.
A. Each point represents a protein, with the x-axis indicating the mean standard deviation (SD) difference in expression after inverse normal transformation (beta value) between the low and high HPS groups and the y-axis representing the statistical significance (-log10 adjusted p-value for multiple testing using Bonferroni correction). Blue points indicate significantly downregulated proteins in the low HPS group (beta < −0.4 and adjusted p-value < 0.05). Red points indicate significantly upregulated proteins in the low HPS group (beta > 0.4 and adjusted p-value < 0.05). Black points indicate proteins that are not significantly differentially expressed. B. The x-axis represents the significant gene sets, ordered by adjusted p-values for multiple testing, and the y-axis shows the -log10 of the adjusted p-value for each significant gene set (Bonferroni-corrected p-value <0.05).
Discussion
Unlike previous biological age predictors trained to predict chronological age, mortality22 or organ/disease-specific predictors23–26, HPS was developed based on a healthspan definition to evaluate the risk of developing a major chronic disease or mortality. HPS outperformed chronological age and other biological age measures in predicting healthspan and various chronic diseases. Subgroup analyses revealed that individuals at higher risk for adverse health outcomes—such as males, older adults, current or former smokers, obese individuals, and those with hypertension or hypercholesterolemia—had lower HPS values, indicating a less healthy systemic biological status in these groups. Additionally, we discovered that the biological processes associated with HPS align with the hallmarks of biological aging, particularly immune response, inflammation, cellular signaling, and metabolic regulation. Overall, our findings demonstrate that HPS has strong clinical, predictive, and biological validity, making it a valuable tool for assessing healthspan in humans and potentially guiding the development of geroscience-based interventions in the future.
Several biological age measures have been developed to predict mortality and the onset of age-related diseases. These measures may reflect different aspects of biological aging due to data variations, study populations, and the methods used for their development8,27. We compared HPS with several well-established biological age measures. HPS and PAC showed a strong correlation (r = −0.87), even after controlling for chronological age (r = −0.65), indicating a significant overlap in their measurements. The correlations of HPS with other biological age measures, such as PhenoAge and BioAge, were less pronounced, and those with leukocyte telomere length and a 49-item frailty index were weak to moderate. Although these measures are strongly associated with mortality and disability, they vary significantly in their ability to predict specific chronic medical conditions. While their measurements may overlap, they also capture distinct facets of the complex biology of aging.
HPS may offer several advantages over other biological age measures, particularly in the context of geroscience-guided interventions. It was trained on relatively young individuals who had not developed chronic medical conditions at baseline and were followed until a first healthspan condition. HPS strongly predicts healthspan, mortality, and the onset of medical conditions in participants free from the conditions in the healthspan definition. Additionally, HPS predicts comorbidities and mortality in those with a specific chronic medical condition. By monitoring the effects of interventions on biological health, HPS can shorten trial lengths by serving as a surrogate measure for healthspan or chronic medical conditions. Therefore, incorporating HPS into clinical trials could enhance the evaluation of interventions to improve overall biological health and prevent chronic diseases.
The joint analysis of HPS and PAC revealed intriguing patterns of associations with healthspan, mortality, and specific chronic medical conditions. Most individuals (70%) were classified in the better biological health group (i.e., high HPS and low PAC). Meanwhile, 19% were in the worse biological health group (i.e., low HPS and high PAC), and a small group, comprising 12% of the sample, were in the intermediate biological health groups (i.e., those with low HPS and low PAC or high HPS and high PAC). As expected, individuals in the worse biological health group showed the highest risk of adverse health outcomes. Those in the intermediate biological health group also had an elevated risk, although the associations were not as strong as those in the worse biological health group. Our findings imply that there is a group of “clinically” healthy individuals who already exhibit significant systemic biological changes, indicating a higher risk of disease development. This group may benefit the most from geroscience-guided interventions, particularly those in transitional stages of biological health (i.e., intermediate biological health groups) who are at higher risks of diseases but could also respond well to such interventions. Prior studies have evaluated proteomic markers associated with chronological age28–30 and longevity, using various definitions of longevity, such as parental age at death above 95 years31 or survival to 90 years32. These studies identified robust proteomic signatures related to chronological age and longevity. Interestingly, a few markers are common across these studies, including ours, such as GDF-15, pleiotrophin, and chordin-like protein 1. Despite differences in proteomic platforms, proteome coverage, statistical modeling, and outcomes (e.g., chronological age, longevity, and healthspan), there appears to be a common proteomic signature that can capture multiple processes involved in healthy aging. Although still elusive, this common signature offers novel opportunities to develop interventions targeting multiple aging-related conditions.
Several limitations should be noted when interpreting our results:
1. Diagnostic Lag:
Disease diagnosis often occurs after the actual onset of pathological processes. Consequently, some participants included in the HPS training set might have already experienced biological changes related to specific chronic medical conditions, which could influence the selection of proteins in the HPS model. To address this issue, we used multi-source data to determine the first diagnosis date, which may reduce the diagnostic lag. Additionally, this diagnostic lag likely occurred randomly, which is expected to drive associations toward the null, meaning our results may be more conservative than the actual associations33.
2. Healthspan definition:
Given that there is no consensus on the healthspan definition, predictors trained using different definitions may differ from HPS in assessing biological aging. We adopted a practical healthspan definition using electronic health records, which differs from the definition used by Walter et al.34. They defined healthspan as the number of years from birth to the first occurrence of conditions in our healthspan definition plus hip fracture. Additionally, a critique of both definitions is that, except for dementia, late-onset diseases are underrepresented, meaning those who remain disease-free may not necessarily be in good health35. Including additional late-onset diseases is unlikely to significantly change our results, as the UKB is a relatively young cohort, and our goal was to train a predictor for early interventions. It is important to note that although our analyses focused on the conditions used to define healthspan, other diseases could also impact healthspan. Therefore, our results do not necessarily reflect a proteomic signature of the complete absence of disease. However, we showed that HPS was a strong predictor of other age-related chronic medical conditions not included in the healthspan definition, indicating that it can be used to assess the risk for multiple chronic conditions.
3. Comparison with Epigenetic Clocks:
Although epigenetic clocks are the most popular biological age predictors, we could not include any for comparison with HPS due to the lack of epigenetic data in the UKB. However, PhenoAge was used to train DNAm PhenoAge, thus providing an indirect comparison between HPS and DNAm PhenoAge clock11.
4. Validation with External Cohorts:
We were unable to validate HPS using external cohorts because no large cohort uses the same proteomic assay as the UKB. Although other cohorts have proteomic data available from assays such as different versions of the Somalogic assay, significant differences in proteome coverage and analytical characteristics prevent us from using these data to validate our models externally.
5. HPS vs. organ/disease-specific clocks:
Despite its robustness, HPS was developed as a tool to test the geroscience hypothesis and may serve as a systemic gauge for monitoring biological health. Recent organ- or disease-specific clocks23–26 may have stronger predictive power for particular diseases of interest. However, HPS may be more useful in studies aimed at monitoring the development of multiple diseases simultaneously.
6. Population Diversity:
The UKB has limited racial and ethnic diversity, being predominantly composed of individuals of Caucasian ancestry. Therefore, our models may not be generalized to more diverse populations.
In conclusion, we have developed a novel healthspan proteomic score termed HPS, which demonstrated robust associations and predictions for healthspan, mortality, and the onset of various diseases. The proteins associated with HPS were enriched in several processes related to the hallmarks of biological aging. HPS can be useful for gauging an individual’s biological health and monitoring the impact of geroscience-guided interventions, serving as a surrogate marker of healthspan.
Online Methods
The UKB is a volunteer community cohort in the United Kingdom that recruited over 500,000 participants aged 40 to 70 between 2006 and 201036. During baseline assessments, data were collected on sociodemographic and lifestyle factors, environmental exposures, health and medical history, self-reported medications, cognitive function, physical activity, physical measurements, and biological samples for future assays. Over the years, various biological data have been generated, including plasma proteomic data from a nested cohort, as part of the UKB PPP37. The baseline cohort participants have been followed up through linkages with electronic health records for disease diagnoses and deaths38.
The UKB PPP participants (initially n=54,219) were primarily a random sample (n=46,595, 85.9%) from the UKB baseline cohort. Additionally, the cohort included individuals selected by the UKB PPP consortium of 13 biopharmaceutical companies with specific disease or ancestry interests (n=6,376) and participants attending the COVID-19 repeat imaging study (n=1,268)37. This study included proteomic data from 53,018 active UKB PPP participants after quality control. Protein concentrations of 2,923 proteins were measured using the Olink Explore 3072 platform and normalized through a two-step process involving within-batch and across-batch intensity normalization. 39. The normalized protein expression (NPX) data were used throughout the present project. Three proteins, GLIPR1 (99.7%), NPM1 (74.0%), and PCOLCE (63.6%), were excluded due to high missing rates. The median individual missing rate was 0.5% (25th percentile 0.1% and 75th percentile 7.5%). However,7.2% of the included samples only had complete proteomic data, necessary for the HPS development methods. To address this issue, we imputed the missing proteomic data using the k-nearest neighbors approach (k=10) with the R package ‘multiUS’40.
Healthspan was defined as the number of years from birth free of cancer (excluding non-melanoma skin cancer), diabetes (including type I diabetes, type II diabetes, and malnutrition-related diabetes), heart failure, MI, stroke, COPD, dementia, or death, in line with previous studies16,17. The UKB acquired the date of death data through linkages with national death registries and derived the first diagnosis dates for conditions in the healthspan definition and other medical conditions based on ICD-10 codes (Supplementary Table 1), which were used to link multi-source data, including primary care records, hospital inpatient data, cancer and death registries records, and self-reported medical condition codes.
Participants without any condition in the healthspan definition at baseline were randomly split into a training set and a test set in a seven-to-three ratio (Supplementary Figure 1). The training set data, including chronological age and NPX of 2920 proteins, was related to time from baseline to a first healthspan condition (censored at death or last follow-up of hospital inpatient data [main source], whichever occurred first) using a LASSO Cox regression model. The death censoring date was 2022/11/30. The inpatient censoring dates were 2022/11/30 for participants attending baseline assessment centers in England, 2021/7/31 for participants in Scotland, and 2018/2/28 for those in Wales. Proteins with non-zero regression coefficients in the fitted model were carried forward to fit a Gompertz regression model. Based on the cumulative density probability of this model, the risk of ending healthspan within 10 years from baseline can be derived (r), with 1-r representing the likelihood of remaining healthy, referred to as the healthspan proteomic score (HPS).
We tested the validity of HPS using participants in the test set data by examining:
Spearman correlations of HPS with chronological age, biological age measures, as well as aging traits at baseline, and between HPS, PAC, and PhenoAge after removing the effect of chronological age in linear regression models, where the composite measures of PAC15, PhenoAge11, BioAge19, and a 49-item frailty index20 were derived using data fields listed in Supplementary Table 1.
Associations of HPS at baseline with healthspan, mortality, and medical conditions during follow-up (detailed in Supplementary Table 1).
Associations of HPS at baseline with the conditions above during follow-up in subgroups by age (≥60, <60), sex (male, female), smoking status (previous or current smokers, never smokers), BMI (≥30, <30), hypertension (yes/no), and hypercholesterolemia (yes/no).
Predictions for the conditions above during follow-up against chronological age, PAC, and PhenoAge
The combined effect of low HPS (HPS<0.73) and high PAC (PAC≥58) versus each alone for associations with the conditions above during follow-up.
In addition, we tested the validity of HPS in those with a specific healthspan condition at baseline for associations with a second healthspan condition and mortality.
The association analysis was conducted using Aalen’s additive hazard models41 adjusting for baseline covariates (sex, ethnicity, education, Townsend deprivation index, BMI, smoking status, hypertension, hypercholesterolemia, and UKB PPP consortium selection, Supplementary Table 1), which facilitated testing for an additive interaction effect between HPS and PAC42. Throughout the association analysis, p-values were adjusted for multiple testing using the Benjamini-Hochberg false discovery rate method43. Harrell’s C statistic44 under a Cox regression model was used to assess the predictive accuracy of HPS versus chronological age, PAC, and PhenoAge for ending healthspan, mortality, and medical conditions during follow-up.
To understand the measurement of HPS, we conducted a gene set enrichment analysis for proteins associated with low HPS (HPS<0.73) after adjusting for baseline covariates. Prior to the association analysis, the inverse normal transformation was applied to each protein to normalize the distribution and unify the scale into z-scores. Proteins significant at the Bonferroni-corrected level were entered into the gene set analysis implemented in the Functional Mapping and Annotation of Genome-Wide Association Studied (FUMA version 1.5.2). Genes associated with HPS were compared with the background genes (20,260 protein-coding genes) for the presence in a hallmark gene set using a hypergeometric test. Enriched hallmark gene sets with at least five genes overlapped with the input genes were identified at the Bonferroni-corrected level of 5% (50 hallmark gene sets in total).
Supplementary Material
Acknowledgments
Access to UK Biobank data was granted under application no. 92647 “Research to Inform the Field of Precision Gerontology” (PI: Richard H. Fortinsky). This research used data assets made available by National Safe Haven as part of the Data and Connectivity National Core Study, led by Health Data Research UK in partnership with the Office for National Statistics and funded by UK Research and Innovation (research which commenced between 1 October 2020–31 March 2021 grant ref MC_PC_20029; 1 April 2021–30 September 2022 grant ref MC_PC_20058). This research also used data provided by patients and collected by the NHS as part of their care and support. Copyright © (year), NHS England. Re-used with the permission of the NHS England [and/or UK Biobank]. All rights reserved.
Funding Information
Access to UK Biobank data was granted under application no. 92647 “Research to Inform the Field of Precision Gerontology” (PI: Richard H. Fortinsky), funded by the Claude D. Pepper Older American Independence Centers (OAIC) program: P30AG067988 (MPIs: George A. Kuchel and Richard H. Fortinsky). CLK, BSD, RHF, and GAK are partially supported by P30AG067988. JLA has a UK National Institute for Health and Care Research (NIHR) Advanced Fellowship (NIHR301844).
Footnotes
Conflict of Interest Statement
We have no conflicting interests to disclose.
Data Availability Statements
Data access is granted upon application to the UK Biobank. The R code (Liu & Kuo n.d.) for computing HPS can be obtained from the GitHub repository at https://github.com/kuo-lab-uchc/HPS.
References
- 1.GHE: Life expectancy and healthy life expectancy [Internet]. [cited 2024 May 21]. Available from: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-life-expectancy-and-healthy-life-expectancy
- 2.Garmany A, Yamada S, Terzic A. Longevity leap: mind the healthspan gap. NPJ Regen Med. 2021. Sep 23;6(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan M, Al Saud H, Sierra F, Perez V, Greene W, Al Asiry S, Pathai S, Torres M. Global Healthspan Summit 2023: closing the gap between healthspan and lifespan. Nat Aging. 2024. Apr;4(4):445–448. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, Richardson A, Schadt EE, Wyss-Coray T, Sierra F. Geroscience: linking aging to chronic disease. Cell. 2014. Nov 6;159(4):709–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuellen G, Jansen L, Cohen AA, Luyten W, Gogol M, Simm A, Saul N, Cirulli F, Berry A, Antal P, Köhling R, Wouters B, Möller S. Health and Aging: Unifying Concepts, Scores, Biomarkers and Pathways. Aging Dis. 2019. Aug;10(4):883–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaeberlein M. How healthy is the healthspan concept? Geroscience. 2018. Aug;40(4):361–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lara J, Cooper R, Nissan J, Ginty AT, Khaw KT, Deary IJ, Lord JM, Kuh D, Mathers JC. A proposed panel of biomarkers of healthy ageing. BMC Med. 2015. Sep 15;13:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belsky DW, Moffitt TE, Cohen AA, Corcoran DL, Levine ME, Prinz JA, Schaefer J, Sugden K, Williams B, Poulton R, Caspi A. Eleven Telomere, Epigenetic Clock, and Biomarker-Composite Quantifications of Biological Aging: Do They Measure the Same Thing? Am J Epidemiol. 2018. Jun 1;187(6):1220–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blodgett JM, Pérez-Zepeda MU, Godin J, Kehler DS, Andrew MK, Kirkland S, Rockwood K, Theou O. Prognostic accuracy of 70 individual frailty biomarkers in predicting mortality in the Canadian Longitudinal Study on Aging. Geroscience. 2024. Jun;46(3):3061–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Z, Kuo PL, Horvath S, Crimmins E, Ferrucci L, Levine M. A new aging measure captures morbidity and mortality risk across diverse subpopulations from NHANES IV: A cohort study. Basu S, editor. PLoS Med. 2018. Dec 31;15(12):e1002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, Hou L, Baccarelli AA, Stewart JD, Li Y, Whitsel EA, Wilson JG, Reiner AP, Aviv A, Lohman K, Liu Y, Ferrucci L, Horvath S. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY). 2018. Apr 18;10(4):573–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, Lu J, Miao J, Li J, Zhu M, Dai J, Ma H, Jin G, Hang D. Development and validation of a blood biomarker score for predicting mortality risk in the general population. J Transl Med. 2023. Jul 15;21(1):471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu AT, Quach A, Wilson JG, Reiner AP, Aviv A, Raj K, Hou L, Baccarelli AA, Li Y, Stewart JD, Whitsel EA, Assimes TL, Ferrucci L, Horvath S. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging. 2019. Jan 21;11(2):303–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deelen J, Kettunen J, Fischer K, van der Spek A, Trompet S, Kastenmüller G, Boyd A, Zierer J, van den Akker EB, Ala-Korpela M, Amin N, Demirkan A, Ghanbari M, van Heemst D, Ikram MA, van Klinken JB, Mooijaart SP, Peters A, Salomaa V, Sattar N, Spector TD, Tiemeier H, Verhoeven A, Waldenberger M, Würtz P, Davey Smith G, Metspalu A, Perola M, Menni C, Geleijnse JM, Drenos F, Beekman M, Jukema JW, van Duijn CM, Slagboom PE. A metabolic profile of all-cause mortality risk identified in an observational study of 44,168 individuals. Nat Commun. 2019. Aug 20;10(1):3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuo CL, Chen Z, Liu P, Pilling LC, Atkins JL, Fortinsky RH, Kuchel GA, Diniz BS. Proteomic aging clock (PAC) predicts age-related outcomes in middle-aged and older adults. Aging Cell. 2024. May 15;e14195. [DOI] [PMC free article] [PubMed]
- 16.Li X, Ploner A, Wang Y, Zhan Y, Pedersen NL, Magnusson PK, Jylhävä J, Hägg S. Clinical biomarkers and associations with healthspan and lifespan: Evidence from observational and genetic data. EBioMedicine. 2021. Apr;66:103318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zenin A, Tsepilov Y, Sharapov S, Getmantsev E, Menshikov LI, Fedichev PO, Aulchenko Y. Identification of 12 genetic loci associated with human healthspan. Commun Biol. 2019;2:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman J, Hastie T, Tibshirani R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J Stat Softw. 2010;33(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- 19.Levine ME. Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age? J Gerontol A Biol Sci Med Sci. 2013. Jun;68(6):667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams DM, Jylhävä J, Pedersen NL, Hägg S. A Frailty Index for UK Biobank Participants. J Gerontol A Biol Sci Med Sci. 2019. Mar 14;74(4):582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Codd V, Denniff M, Swinfield C, Warner SC, Papakonstantinou M, Sheth S, Nanus DE, Budgeon CA, Musicha C, Bountziouka V, Wang Q, Bramley R, Allara E, Kaptoge S, Stoma S, Jiang T, Butterworth AS, Wood AM, Di Angelantonio E, Thompson JR, Danesh JN, Nelson CP, Samani NJ. Measurement and initial characterization of leukocyte telomere length in 474,074 participants in UK Biobank. Nat Aging. 2022. Feb;2(2):170–179. [DOI] [PubMed] [Google Scholar]
- 22.Moqri M, Herzog C, Poganik JR, Biomarkers of Aging Consortium, Justice J, Belsky DW, Higgins-Chen A, Moskalev A, Fuellen G, Cohen AA, Bautmans I, Widschwendter M, Ding J, Fleming A, Mannick J, Han JDJ, Zhavoronkov A, Barzilai N, Kaeberlein M, Cummings S, Kennedy BK, Ferrucci L, Horvath S, Verdin E, Maier AB, Snyder MP, Sebastiano V, Gladyshev VN. Biomarkers of aging for the identification and evaluation of longevity interventions. Cell. 2023. Aug 31;186(18):3758–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh HSH, Rutledge J, Nachun D, Pálovics R, Abiose O, Moran-Losada P, Channappa D, Urey DY, Kim K, Sung YJ, Wang L, Timsina J, Western D, Liu M, Kohlfeld P, Budde J, Wilson EN, Guen Y, Maurer TM, Haney M, Yang AC, He Z, Greicius MD, Andreasson KI, Sathyan S, Weiss EF, Milman S, Barzilai N, Cruchaga C, Wagner AD, Mormino E, Lehallier B, Henderson VW, Longo FM, Montgomery SB, Wyss-Coray T. Organ aging signatures in the plasma proteome track health and disease. Nature. 2023. Dec 7;624(7990):164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goeminne LJE, Eames A, Tyshkovskiy A, Argentieri MA, Ying K, Moqri M, Gladyshev VN. Plasma-based organ-specific aging and mortality models unveil diseases as accelerated aging of organismal systems [Internet]. 2024. [cited 2024 May 24]. Available from: 10.1101/2024.04.08.24305469 [DOI] [PubMed]
- 25.Sehgal R, Meer M, Shadyab AH, Casanova R, Manson JE, Bhatti P, Crimmins EM, Assimes TL, Whitsel EA, Higgins-Chen AT, Levine M. Systems Age: A single blood methylation test to quantify aging heterogeneity across 11 physiological systems [Internet]. 2023. [cited 2024 Apr 4]. Available from: 10.1101/2023.07.13.548904 [DOI] [PubMed]
- 26.You J, Guo Y, Zhang Y, Kang JJ, Wang LB, Feng JF, Cheng W, Yu JT. Plasma proteomic profiles predict individual future health risk. Nat Commun. 2023. Nov 28;14(1):7817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrucci L, Gonzalez-Freire M, Fabbri E, Simonsick E, Tanaka T, Moore Z, Salimi S, Sierra F, de Cabo R. Measuring biological aging in humans: A quest. Aging Cell. 2020. Feb;19(2):e13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menni C, Kiddle SJ, Mangino M, Viñuela A, Psatha M, Steves C, Sattlecker M, Buil A, Newhouse S, Nelson S, Williams S, Voyle N, Soininen H, Kloszewska I, Mecocci P, Tsolaki M, Vellas B, Lovestone S, Spector TD, Dobson R, Valdes AM. Circulating Proteomic Signatures of Chronological Age. J Gerontol A Biol Sci Med Sci. 2015. Jul;70(7):809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka T, Biancotto A, Moaddel R, Moore AZ, Gonzalez-Freire M, Aon MA, Candia J, Zhang P, Cheung F, Fantoni G, CHI consortium, Semba RD, Ferrucci L. Plasma proteomic signature of age in healthy humans. Aging Cell. 2018. Oct;17(5):e12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehallier B, Gate D, Schaum N, Nanasi T, Lee SE, Yousef H, Moran Losada P, Berdnik D, Keller A, Verghese J, Sathyan S, Franceschi C, Milman S, Barzilai N, Wyss-Coray T. Undulating changes in human plasma proteome profiles across the lifespan. Nat Med. 2019. Dec;25(12):1843–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sathyan S, Ayers E, Gao T, Weiss EF, Milman S, Verghese J, Barzilai N. Plasma proteomic profile of age, health span, and all-cause mortality in older adults. Aging Cell. 2020. Nov;19(11):e13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X, Axelsson GT, Newman AB, Psaty BM, Boudreau RM, Wu C, Arnold AM, Aspelund T, Austin TR, Gardin JM, Siggeirsdottir K, Tracy RP, Gerszten RE, Launer LJ, Jennings LL, Gudnason V, Sanders JL, Odden MC. Plasma proteomic signature of human longevity. Aging Cell. 2024. Mar 5;e14136. [DOI] [PMC free article] [PubMed]
- 33.Loken E, Gelman A. Measurement error and the replication crisis. Science. 2017. Feb 10;355(6325):584–585. [DOI] [PubMed] [Google Scholar]
- 34.Walter S, Atzmon G, Demerath EW, Garcia ME, Kaplan RC, Kumari M, Lunetta KL, Milaneschi Y, Tanaka T, Tranah GJ, Völker U, Yu L, Arnold A, Benjamin EJ, Biffar R, Buchman AS, Boerwinkle E, Couper D, De Jager PL, Evans DA, Harris TB, Hoffmann W, Hofman A, Karasik D, Kiel DP, Kocher T, Kuningas M, Launer LJ, Lohman KK, Lutsey PL, Mackenbach J, Marciante K, Psaty BM, Reiman EM, Rotter JI, Seshadri S, Shardell MD, Smith AV, van Duijn C, Walston J, Zillikens MC, Bandinelli S, Baumeister SE, Bennett DA, Ferrucci L, Gudnason V, Kivimaki M, Liu Y, Murabito JM, Newman AB, Tiemeier H, Franceschini N. A genome-wide association study of aging. Neurobiol Aging. 2011. Nov;32(11):2109.e15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deelen J. Targeting multimorbidity: Using healthspan and lifespan to identify biomarkers of ageing that pinpoint shared disease mechanisms. EBioMedicine. 2021. May;67:103364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, Liu B, Matthews P, Ong G, Pell J, Silman A, Young A, Sprosen T, Peakman T, Collins R. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015. Mar;12(3):e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun BB, Chiou J, Traylor M, Benner C, Hsu YH, Richardson TG, Surendran P, Mahajan A, Robins C, Vasquez-Grinnell SG, Hou L, Kvikstad EM, Burren OS, Davitte J, Ferber KL, Gillies CE, Hedman ÅK, Hu S, Lin T, Mikkilineni R, Pendergrass RK, Pickering C, Prins B, Baird D, Chen CY, Ward LD, Deaton AM, Welsh S, Willis CM, Lehner N, Arnold M, Wörheide MA, Suhre K, Kastenmüller G, Sethi A, Cule M, Raj A, Alnylam Human Genetics, AstraZeneca Genomics Initiative, Biogen Biobank Team, Bristol Myers Squibb, Genentech Human Genetics, GlaxoSmithKline Genomic Sciences, Pfizer Integrative Biology, Population Analytics of Janssen Data Sciences, Regeneron Genetics Center, Kang HM, Burkitt-Gray L, Melamud E, Black MH, Fauman EB, Howson JMM, Kang HM, McCarthy MI, Nioi P, Petrovski S, Scott RA, Smith EN, Szalma S, Waterworth DM, Mitnaul LJ, Szustakowski JD, Gibson BW, Miller MR, Whelan CD. Plasma proteomic associations with genetics and health in the UK Biobank. Nature. 2023. Oct 12;622(7982):329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allen NE, Lacey B, Lawlor DA, Pell JP, Gallacher J, Smeeth L, Elliott P, Matthews PM, Lyons RA, Whetton AD, Lucassen A, Hurles ME, Chapman M, Roddam AW, Fitzpatrick NK, Hansell AL, Hardy R, Marioni RE, O’Donnell VB, Williams J, Lindgren CM, Effingham M, Sellors J, Danesh J, Collins R. Prospective study design and data analysis in UK Biobank. Sci Transl Med. 2024. Jan 10;16(729):eadf4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun B, Ferber K, Lin T, Whelan C. UK Biobank Pharma Proteomics Project: Olink quality control summary [Internet]. [cited 2024 Jun 2]. Available from: https://biobank.ndph.ox.ac.uk/ukb/refer.cgi?id=4658
- 40.Torgo L. Data Mining with R [Internet]. 0 ed. Chapman and Hall/CRC; 2011. [cited 2023 Aug 18]. Available from: https://www.taylorfrancis.com/books/9781439876404
- 41.Aalen O. A Model for Nonparametric Regression Analysis of Counting Processes. In: Klonecki W, Kozek A, Rosiński J, editors. Mathematical Statistics and Probability Theory [Internet]. New York, NY: Springer New York; 1980. [cited 2024 Jun 3]. p. 1–25. Available from: 10.1007/978-1-4615-7397-5_1 [DOI] [Google Scholar]
- 42.Rod NH, Lange T, Andersen I, Marott JL, Diderichsen F. Additive Interaction in Survival Analysis: Use of the Additive Hazards Model. Epidemiology. 2012. Sep;23(5):733–737. [DOI] [PubMed] [Google Scholar]
- 43.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society: Series B (Methodological). 1995. Jan;57(1):289–300. [Google Scholar]
- 44.Harrell FE, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982. May 14;247(18):2543–2546. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data access is granted upon application to the UK Biobank. The R code (Liu & Kuo n.d.) for computing HPS can be obtained from the GitHub repository at https://github.com/kuo-lab-uchc/HPS.