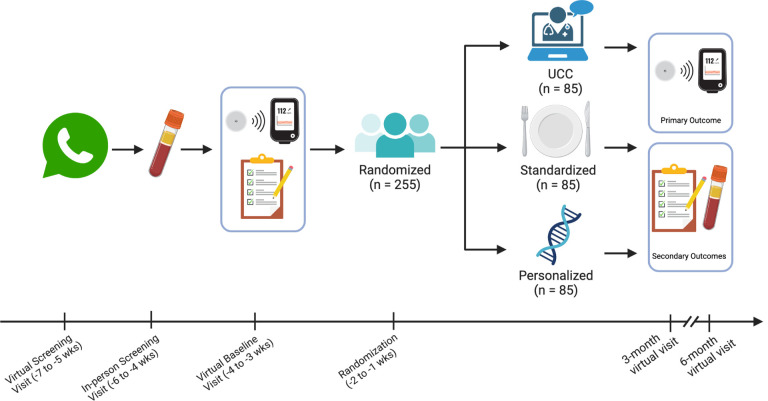
Figure 1.
Overview of study design and measurement timepoints
Patients who are interested in enrolling in the study complete a virtual screening to determine eligibility. Eligible participants are scheduled for an in-person blood draw at a Quest Laboratory location of their choice to confirm HbA1c eligibility requirements before participating in study activities. Prior to measurement timepoints, participants are mailed CGM sensors and a CGM reader. At each measurement timepoint, participants complete electronic questionnaires, wear a CGM sensor for 12 days, and visit a Quest Laboratory location for an in-person blood draw. After the baseline assessment, participants are randomized to one of three study intervention arms: UCC, Standardized, or Personalized.Additional measurements occur at 3 and 6 months.