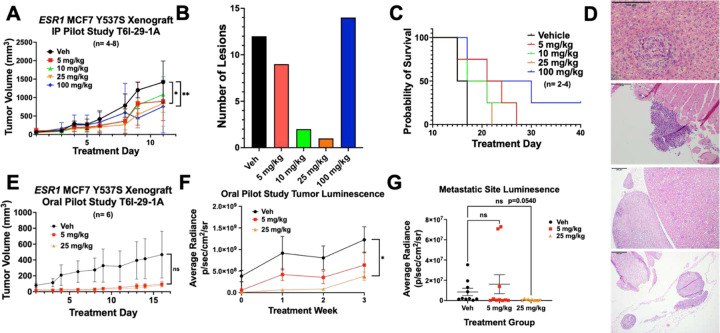
Figure 5:
T6I-29-1A inhibits tumor growth in preliminary in vivo studies. A) Tumor growth (error bars indicate SEM) in I.P. pilot study, n= 4–8 tumors/ group. Significance is measured by Two-Way Anova with Bonferroni post-hoc test, results indicate day 9 treatment analysis. B) Total metastatic lesions as measured by H&E staining by Dr. Khin Su Mon across groups. C) Survival curve of I.P. pilot study, significance determined using log rank test. Veh vs 100 mg/kg p=0.0624. D) Representative photos capturing metastases (top to bottom) in liver (vehicle treated), left femur (vehicle treated), adrenal gland (100 mg/kg treated), and uterus (vehicle treated). E) Tumor growth (error bars indicate SEM) in oral pilot study, n= 6 tumors/ group. Analyzed with Two-Way Anova with Bonferroni post-hoc test. F) Tumor luminescence of oral pilot study measured weekly (error bars indicate SEM). Analyzed with unpaired t-test at treatment week 3. G) Luminesence of liver, lung, brain, femurs, uterus were measured for each mouse in each group ex vivo (error bars indicate s.d.), results were graphed based on treatment groups, including both sides of organ luminescent signal. Anova with Tukey post-hoc statistical test was used to determine significance.