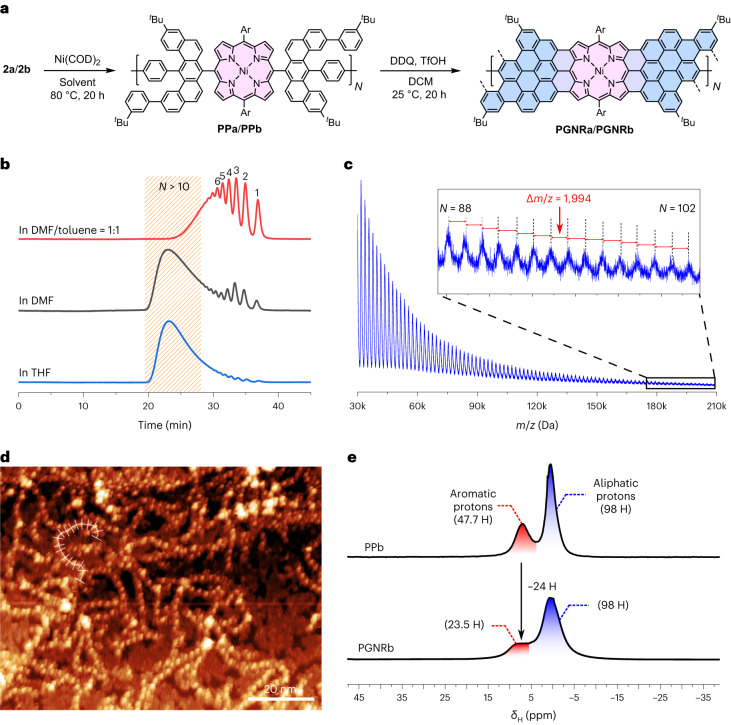
Fig. 4. Synthesis and structural characterization of PGNRs.
a, Synthesis of PGNRs, with the porphyrin and graphene nanoribbon moieties highlighted in pink and blue, respectively. b, Analytical GPC traces of Yamamoto polymerization products of dichloroporphyrin 2b from reaction in DMF/toluene (1:1, v/v), DMF and THF (eluent: THF/1% pyridine, flow rate = 1 ml min–1, detection at 430 nm). Peaks are labelled with N, the number of repeat units. c, MALDI-TOF mass spectrum of PPb measured with trans-2-[3-(4-tert-butylphenyl)-2-methyl-2-propenylidene]malononitrile (DCTB) as the matrix, conducted in the linear mode. Peaks from the polymer extend up to a molecular weight of 210 kDa (102 repeat units) and the average m/z difference between neighbouring peaks corresponds to one repeat unit. d, STM topograph of PPa transferred from toluene solution to a Au(111) surface via electro-spray deposition, and subsequently annealed to 250 °C (Tsample = 4.7 K, Vsample-bias = 2 V, Iset-point = 50 pA). e, CP-MAS solid-state 1H-NMR spectra of PPb in comparison with PGNRb, and the integration of protons in the aromatic and aliphatic regions.