FIGURE 5.
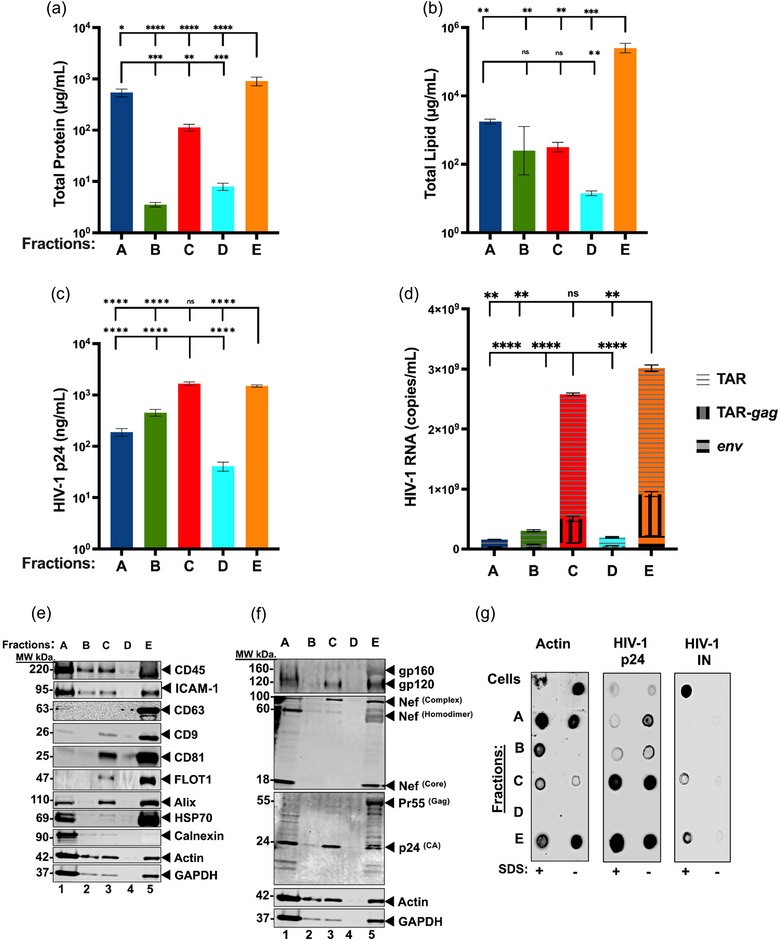
Biochemical content analysis of J1.1LAV EPs. (a) Micro BCA total protein content (µg/mL); (b) Total lipid assay (µg/mL); (c) HIV‐1 p24‐antigen capture ELISA (pg/mL); (d) Cumulative RT‐qPCR for HIV‐1 RNA TAR TAR‐gag (copies/mL); (e) J1.1LAV EPs fractions western blotting (WB) probed for T‐cell marker (CD45), cell adhesion (ICAM‐1), extracellular vesicle tetraspanins markers (CD63, CD9, and CD81), extracellular cargo marker (TSG101, Alix, HSP70, Actin); (f) J1.1LAV EPs fractions WB for HIV‐1 protein content (gp120, Nef, and p24); (g) J1.1LAV EPs SDS membrane protection assay dot blotting of treated or not treated fractions probed for the presence Actin and HIV‐1 markers p24, Integrase (IN). For each measurement technical triplicates were executed. Data represents mean ± standard deviation (SD) of three technical replicate measurements. Statistical significance was calculated with One‐way ANOVA with Tukey's post‐hoc analysis multiple comparisons test with *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 significance level.
