Abstract
Alzheimer’s disease (AD) is a serious dementia afflicting aging population and is characterized by cognitive decline, amyloid-β plaques, and neurofibrillary tangles. AD substantially impairs the life quality of the victims and poses a heavy burden on the society at large. The number of people with dementia due to AD, prodromal AD, and preclinical AD is estimated to stand at roughly 3.2, 69, and 315 million worldwide, respectively. Current clinical diagnosis is based on clinical symptoms, and clinical research demonstrated that positron emission tomography (PET) and cerebrospinal fluid (CSF) biomarkers had excellent diagnostic performance. However, the application of CSF biomarker tests and PET are restricted by the invasiveness and high cost. The presence of clinical symptoms means that AD pathology has been progressing for many years, and only a few drugs have been approved for the traetemnt of AD. Therefore, early diagnosis is extremely important for controlling the outcomes caused by AD. In this review, we provided an overview of developing clinical diagnostic criteria, diagnostic strategies under clinical research, developing blood based-biomarker assays, and promising nanotechnologically-based assays.
Keywords: Alzheimer’s Disease, the National Institute on Aging and Alzheimer’s Association, Diagnostic criteria, Detection methods, Biomarker
1 INTRODUCTION
Alzheimer’s disease is caused by damage of neurons in brain, with neurons in areas responsible for memory and language being involved first, and then areas responsible for basic selfcare functions, such as mobility, affected, thereby presenting a life-threatening risk to the victim. AD is a progressive condition, the rate of progression varying with different individuals [1]. Since the disease is most common in people over 65, the initial onset of the disease, such as memory loss, is not easily noticed by patients and their families, leading to lost opportunities of timely diagnosis and pharmacological interventions. The etiology of the disease is not yet fully understood. Known risk factors include age, genes, and family history, with age being the most important risk factor. The percentage of people with AD increases with age, with up to 33.3% of people over 85 suffering from AD [2]. Among the genetic factors, APOE is most striking, with three allelic mutants, i.e., APOE-e2, APOE-e3, and APOE-e4. APOE-e4 greatly increases the risk of AD development by driving amyloid pathology [3]. A study showed that 65% of 1770 Alzheimer’s patients in the United States had at least one copy of the APOE-e4 gene [4]. In addition to the aforementioned non-modifiable factors, unhealthy habits, such as smoking, may increase the risk of AD [5], and it is possible that an overall reduction in the incidence of smoking may reduce the future prevalence of AD in the population. Additionally, it is still being investigated whether changes of daily dietary habits contribute to a reduction in the incidence of AD [6-7].
Since AD was discovered in the last century, so far, there is no cure for the condition due to the complexity of its pathogenesis, and the knowledge about AD is constantly being updated [8]. With regard to the AD diagnosis, the Alzheimer’s Disease and Related Disorders Association (ADRDA) and the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) proposed the first clinical diagnostic criteria for AD in 1984 [9], and the clinical diagnostic criteria were to be revised 27 years later by the National Institute on Aging and Alzheimer’s Association (NIA-AA) in 2011 [10-13]. With the two versions, the diagnosis mainly depends on patients’ clinical symptoms and cognitive tests. In 2018, a research framework was formulated by the NIA-AA. The framework, for the first time, provided a biological definition of AD and suggested how biomarkers should be used to guide the diagnosis of AD [14]. In 2023, the Alzheimer’s Association released a draft of revised diagnostic criteria with specific guidance on the use of biomarkers for the diagnosis of AD. In the discusssion of the diagnostic methods, this review focused more on the detection of biomarkers than on clinical symptoms. At present, biomarker assays commonly used in clinical research include enzyme-linked immunosorbent assay (ELISA), single-molecule array (Simoa), PET, among others. Here, we review the process of establishing clinical diagnostic criteria and the diagnostic approaches currently under use and development.
2 DIAGNOSTIC CRITERIA AND RESEARCH FRAMEWORK
2.1 Clinical diagnostic criteria established in 1984
In 1984, a Work Group, set up by NINCDS and ADRDA, drafted the clinical diagnosis criteria that defined AD in terms of clinicopathological features. The clinical criteria outlined the diagnosis of probable, possible, and definite AD. Due to the lack of knowledge about the disease, these diagnostic criteria are not fully authoritative and need to be validated and revised on the basis of accumulating clinical experience [9]. After ruling out other neurological disorders (e.g., Parkinson’s disease, bipolar disorder) that may lead to cognitive deficits, the diagnostic criteria propose that a diagnosis of probable AD can be made if the patient presents with typical symptoms of latent dementia, such as sudden apoplectic onset, focal neurologic findings, gait disturbances, etc. [15]. A diagnosis of definite AD requires a combination of medical history, neurologic, psychiatric and clinical examinations, neuropsychological tests, and laboratory studies. Laboratory assessments include electrophysiological methods, computerized tomography (CT), regional cerebral blood flow, PET, magnetic resonance imaging (MRI), and examination of body fluids and non-neurological tissues. These laboratory assessments are used to eliminte other causes of dementia and improve diagnostic accuracy [9]. For example, CT is used to exclude hydrocephalus, brain tumors, and identify abnormal brain tissue changes. Localized cerebral blood flow measurements help to differentiate between dementia due to cerebrovascular diseases and AD [16].
2.2 Clinical diagnostic criteria updated in 2011
In the intervening 27 years, the diagnostic criteria established in 1984 were universally adopted by clinicians. With incremental clinical experience and advances in diagnostic technologies, the diagnostic criteria received a major update in 2011. Compared with the diagnostic criteria published in 1984, there were two significant differences between the two versions: the formal identification of different stages of the disease and the rational use of biomarkers in different states. The diagnostic criteria published in 1984 assumed that the clinical symptoms of AD closely corresponded to the progression of the pathology, and no distinction was made between AD pathology and clinical symptoms. Under the influence of this view, it was believed that individuals either developed AD pathology and were in the dementia category or did not have AD pathology and AD causing dementia could be excluded. Over the following two decades, however, physicians, in their clinical practice, encountered symptoms that were not always consistent with pathology, such as diffuse amyloid plaques detected in the absence of significant clinical symptoms [17]. Therefore, in this revision, a conceptual distinction was made between the physiological process of AD (abbreviated as AD-P) and the resulting clinical syndrome (abbreviated as AD-C). Mild cognitive impairment (MCI) and genetic risk are also emphasized in this revision [11].
The preclinical phase of AD is divided into three stages: the first state features asymptomatic cerebral amyloidosis but positive amyloid β (Aβ) (PET or CSF), the second state has asymptomatic amyloidosis and “downstream” neurodegeneration, with positivity for Aβ (PET or CSF) and markers of neuronal damage (tau, fludeoxyglucose (FDG), sMRI), and the final stage presents with amyloidosis, neuronal damage, and subtle cognitive/ behavioural decline, positivivty for Aβ (PET or CSF) and markers of neuronal damage (tau, FDG, sMRI), and evidence of subtle cognitive changes. The importance of the preclinical stage cannot be overstated, as several studies have shown that the pathological process of AD begins years before the onset of symptoms, meaning that the onset of AD-P precedes the appearance of AD-C. Intervention in the early stages of AD contributes to treatment of disease. The Work Group is committed to the characterization of the preclinical stages of AD using biomarkers [13].
To determine MCI status in AD, two sets of criteria were put forward: (1) core diagnostic criteria, which do not require the use of advanced imaging techniques or CSF analysis, and (2) research criteria, including the use of biomarkers based on imaging and CSF analysis, can be used in clinical studies. The core clinical criteria include cognitive changes, objective manifestations of impairment in cognitive domains, normal functional ability, absence of dementia, and adequate etiological investigation. Different from its core counterpart, research criteria used biomarkers reflecting Aβ deposition (CSF Aβ42, PET amyloid imaging), neuronal injury (CSF tau/p-tau, hippocampal volume or medial temporal atrophy, brain atrophy rate, FDG-PET imaging, Single-Photon Emission Computed Tomography (SPECT) perfusion imaging, etc.) and associated biochemical changes (biomarkers of inflammation, oxidative stress and other markers of neurodegeneration and synaptic damage). More clinical experience is needed to support how these biomarkers can be utilized to provide an objective diagnostic basis. In addition, the Work Group recommends the core diagnostic criteria be combined with biomarker testing to determine whether a patient’s MCI is caused by AD [10].
For dementia due to AD, the core diagnostic criteria developed by the Work Group do not rely on biomarkers, but mainly on clinical symptoms. The Work Group also outlines the core diagnostic criteria for all-cause dementia, which primarily include assessment of daily behaviors and function, and assessment of cognitive and behavioral impairments, such as information acquisition, complex task processing, visuospatial abilities, language functioning, and mood changes. Impairment of daily activities is the main feature that distinguishes between dementia and MCI. AD dementia can be categorized as probable AD dementia, possible AD dementia, and probable or possible AD dementia with the presence of the AD pathological process. To support a diagnosis of AD dementia on the basis of biomarkers, the core clinical diagnostic criteria for AD dementia must be met first [12].
In summary, biomarkers have begun to receive attention when they were included as an adjunct of AD’s diagnosis in the 2011 version of the diagnostic criteria, and are being increasingly used in clinical studies and judgement of the pathological process of AD. Alzheimer’s diagnosis on the basis of biomarkers warrants more clinical experience.
2.3 Research framework established in 2018
In 2018, a research framework was established to provide a biological definition of AD. The mounting importance of biomarkers was the primary impetus to the formulation of the research framework in 2018. In the framework, biomarkers are categorized as Aβ deposition (abbreviated as A, including Aβ-PET and CSF Aβ42), pathological tau (abbreviated as T, including p-tau and tau-PET), and neurodegeneration (abbreviated as N, including T-tau, FDG-PET, and MRI). The classification is not final but flexiable to allow for addition of new biomarkers when they are shown to reflect the pathology of AD [14].
In this framework, AD is defined by Aβ plaques and pathological Tau deposits, and AD is viewed as a continuum of biomarker changes. If only amyloid beta deposits are present and levels of pathological tau biomarkers are normal, the patient will be labeled as “pathological changes in Alzheimer’s disease” rather than “Alzheimer’s disease”. Patients can only be labeled as “Alzheimer’s disease” when both Aβ and pathological tau are abnormal. Neurodegeneration (N) is not specific to AD, so N is only used to assess the severity of the disease [14].
Based on the foregoing conception, the Work Group, commissioned by NIA-AA leadership, stressed that the research framework should not be used to restrict clinical diagnosis. Clinical research is the main target of the research framework. Nevertheless, differing perspectives emerge after the publication of the research framework. Jack et al. compared the prevalence of AD using the biological definition of AD and the clinical definition of probable AD in the same cohort. Their results showed that the prevalence of biologically defined AD was higher than the prevalence of clinically defined AD because changes in biomarkers preceded clinical symptoms, meaning that AD is not the same under the two evaluation modalities, which can create potential confusion in the definition of AD [18]. Ron Louie expressed the same concern, he also supported that defining “Alzheimer’s disease” in terms of biomarkers is hasty, which creates ambiguity in the original clinicopathological definition of “Alzheimer’s disease” [19]. Kevin et al. focused on the “grey space” around the quantitative thresholds for biomarker diagnosis, which should be evaluated in a more comprehensive and multidimensional way [20]. Tang et al. gave a similar view [21].
Over the past few years, researchers have shown great enthusiasm for the biomarkers of AD, and several studies demonstrated the accuracy of biomarkers for Alzheimer’s diagnosis [22-23].
2.4 Revised criteria for Diagnosis of AD: A Draft
In 2022, the Alzheimer’s Association formed a steering committee and a Work Group led by Dr. Clifford Jack to review the 2011 diagnostic criteria and the 2018 clinical research framework and then updated the diagnostic criteria. The Alzheimer’s Association released a draft of revised diagnostic criteria in October 2023 and is currently reviewing comments solicited from the scientific community to publish revised diagnostic criteria in 2024 [24].
The primary rationale for this update is that biomarkers were widely validated in clinical research, so the use of biomarkers for Alzheimer’s diagnosis should go one step further. Several basic principles of this revision include: (1) AD should be defined biologically rather than based on clinical symptoms; (2) AD is a continuum in which progressive brain pathological changes lead to the development of clinical symptoms; (3) AD should be diagnosed on the basis of abnormalities in biomarkers [25].
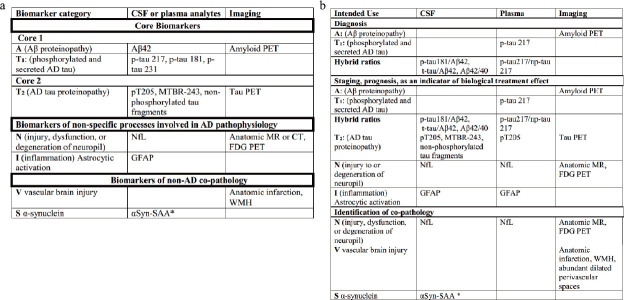
In this draft, the classification of biomarkers is further refined, a distinction was made between the same biomarker on fluid assays and imaging assays, where biomarker imaging measures the cumulative effect of the biomarker and fluid assays reflect the production/clearance rate of the analyte. Based on the 2018 ATN classification, biomarkers of inflammatory/immune mechanisms (I), vascular brain injury biomarkers (V), and synucleinopathy biomarkers (S) were introduced, and these biomarkers, classified in terms of underlying mechanisms, were categorized into core AD biomarkers, biomarkers of non-specific processes involved in AD pathophysiology, and biomarkers of non-AD co-pathology [26]. The specific classification and application of these biomarkers are presented in Figure 1.
Figure 1.
Classification (a) and application (b) of biomarkers for AD diagnosis in the draft [26].
This is the first diagnostic guideline for biomarkers used in clinical diagnosis, but the Work Group also clarified that this guideline should serve as a bridge between research and clinical application, meaning it is not intended to be a specific guideline for clinical practice. Since the revised diagnostic criteria have not yet been released in an official version, we will not discuss them in more detail, but we can find that clinial application of biomarkers is going forward with the continuous updating of the diagnostic criteria, and biological tests are the basis for the application of biomarkers as a reliable diagnostic tool, as we will discuss in the following sections.
3 BIOLOGICAL DIAGNOSTIC APPROACH
3.1 CSF Aβ42/40 and p-tau
CSF is in direct contact with brain and spinal cord, so its protein composition is close to that of the brain tissue, making CSF an ideal source of biomarkers for AD and can be used to characterize changes in biochemical markers in the brain [27]. Amyloid precursor protein (APP) in the brain is concentrated in neuronal synapses and can be degraded into different products by a variety of proteases. Aβ42 is prone to accumulation and formation of deposits that can lead to neurotoxicity. Therefore, Aβ plaques are considered to be the main pathological marker of AD [28-30]. In fact, AD can be recognized by a decreased Aβ42 level in CSF. Studies have shown that level of Aβ42 was abnormal decades before the onset of dementia, suggesting that level of Aβ42 is conducive to the early diagnosis and an early warning sign of AD. In addition, a study has shown that Aβ42/40 outperformed Aβ42 in terms of diagnostic accuracy [31].
Tau protein is mainly found in axons of the central nervous system (CNS) and plays an important role in the stabilization of microtubules, which is crucial for neuronal integrity. Phosphorylation of tau impairs the affinity of tau with microtubules and thus causes microtubule disassembly, which in turn affects the integrity of neurons. Excessively phosphorylated tau clusters together to form helical filaments (PHFs) and straight filaments. Neurofibrillary tangles (NFTs) formed by PHFs is another fundamental pathological feature of AD. The level of total tau in CSF reflects the severity of neuronal degeneration, but the elevation of t-tau is not found solely in AD patients [32-34]. In contrast, some studies have shown that p-tau levels were elevated only in AD, with no or minimal changes in other neurodegenerative disorders, such as traumatic brain injury, stroke, and Creutzfeldt-Jakob disease [35]. Levels of p-tau181, p-tau217, and p-tau231 rose when only subtle Aβ pathology was detected in the preclinical Alzheimer’s continuum [36]. The levels of p-tau in CSF increased significantly, allowing for an accurate differentiation between Aβ-positive and Aβ-negative populations. Given the invasiveness of lumbar puncture and the immaturity of diagnostic application of biomarkers, the Work Group made recommendations the possible indications for the use of CSF biomarkers as an aid to diagnosis [37]. ELISA is widely used as a high-throughput, convenient, and sensitive method for the detection of AD biomarkers in CSF [38-39].
3.2 Aβ-PET/tau-PET
PET is a non-invasive imaging technique commonly used for clinical diagnosis and in scientific research. Based on the decay process of radioisotopes, PET visualizes metabolic and functional structure in the brain by detecting positrons emitted from radioisotopes to generate images. The radiotracer 18F-fluorodextrose (FDG-PET) is commonly used for the visualization of the brain, and patients with AD or non-AD dementias exhibit hypometabolic features. The distribution pattern of hypometabolism determines the type and severity of dementias. Typical FDG-PET manifestations in AD patients include reduced glucose metabolism in the temporoparietal joint cortex, anterior cingulate, and posterior cingulate [40]. The specificity of the radiotracer is extremely important for the imaging of specific metrics. 11C-Pittsburgh compound B (11C-PIB) is the first tracer to be used for Aβ-PET, and UC-PIB binds to the β-sheet structure, especially fibrotic Aβ, but the short half-life of C-11 limits its more widespread use. To overcome this problem, fluorine-18 labeled radiotracers were developed and introduced and they include [18F]florbetapir, [18F] flutemetamol, and [18F]florbetaben, and have been approved by the FDA for clinical use. They have exhibited great potential in distinguishing patients with AD from the healthy population as well as in the early detection of AD progression [41-42].
Prior studies have shown that early tau deposition is confined to the transnasal medial region, then spreads to the limbic lobes, and finally to the neocortex [43]. Therefore, tau-PET can help physicians determine the progression of the disease. Since the concentration of tau aggregates in brain tissue is much lower than that of Aβ, tracer specificity has to be high. Currently, 18FAV-1451 is widely used, and has been shown to possess a high affinity for PHF-tau, but binds poorly with other biomarkers, such as Aβ, argyrophilic grains, and α-synuclein, so it can serve to differentiate AD and other non-AD degenerative diseases [44-45]. The distribution of tracers can be classified according to the pattern specified by Braak, thus helping physicians assess the pathological progression of patients [46]. The 18F-AV-1451 is being increasingly applied in the clinical practice [47].
3.3 Detection of Blood-Based Biomarkers
As mentioned above, two AD biomarkers, Aβ42 and p-tau can be detected by CSF sampling and PET imaging, but CSF sampling entails lumbar puncture, which is extremely painful for patients, and PET imaging is expensive [48], so research on blood-based biomarkers has been gaining momentum in recent years. Peripheral blood sampling is convenient, which can help facilitate the further clinical application of AD biomarkers. Currently, representative biomarkers in peripheral blood include Aβ42/40, p-tau181, p-tau231, and p-tau217, and the non-specific biomarkers NfL and GFAP [49].
Biomarkers in CSF come directly from the brain, and their concentrations can often reach hundreds of picogram per milliliter, but when these biomarkers enter the bloodstream through systemic circulation, their concentrations tend to drop to a few tens of picogram per milliliter. Therefore, ELISA, which is commonly used for the detection of biomarkers in CSF, is ill-suited for the detection of these biomarkers in blood [50]. Based on the double-antibody sandwich principle, Simoa technology binds approximately 250000 capture antibodies to the 2.7-μm magnetic beads, which are then sequentially conjugated to the antigen, biotinylated secondary antibody, and reaction substrate. After binding, the beads are enclosed in a chip with 238,000 wells, each well containing only one bead, and the fluorescence is detected by a Charge-Coupled Device (CCD) system. The protein concentration corresponding to positive fluorescent wells can be calculated according to the theory of Poisson distribution, thereby meeting the goal of digital single-molecule detection. The Simoa method has two significant advantages: (1) an ultra-low reaction system, which means lower background noise and signal spreading while increasing sensitivity; and (2) a digital assay design and quantification method that allows for independent identification and calculation of single-molecule signals. Compared with ELISA, Simoa has a thousands-fold lower limit of detection (LOD) and limit of quantitation (LOQ), making it possible to accurately detect AD biomarkers in the blood [51-52].
Meso Scale Discovery (MSD) is also an ultrasensitive assay based on a double-antibody sandwich assay with electrochemiluminescence. Unlike ELISA, its detection antibody is labeled with ruthenium instead of biotin, and the bottom of the MSD-specific well plate is a carbon electrode. After the formation of the capture antibody-antigen-detection antibody complex, the ruthenium on the detection antibody reacts with Ru(bpy)32+ and tripropylamine (TPA) to produce an intense light that can be detected at a wavelength of 620 nm. MSD showed an ultra-high sensitivity, and up to 10 indicators can be detected simultaneously in one sample well which includes 10 small wells [53]. Immunoprecipitation mass spectrometry is another option, and with this technique, beads coupled with antibodies separate the detected substance from the sample, then detected substance was eluted, and isotopically labeled substance was finally quantified by mass spectrometrical methods [48].
Several studies compared plasma Aβ42/Aβ40 with CSF Aβ42/Aβ40 and Aβ-PET results using Simoa and Immunoprecipitation mass spectrometry respectively. It was found that plasma Aβ42/Aβ40 was highly correlated with CSF Aβ42/Aβ40 and Aβ-PET results [54-56]. Compared with healthy individuals, Aβ42/Aβ40 in CSF was about 50% lower in patients presenting Aβ pathology while the corresponding ratio in plasma was only 14.3% lower, which might be ascribed to the fact that plasma Aβ contains a fraction of Aβ from the peripheral tissues and Aβ42 is metabolized more rapidly in the blood [57]. Among a variety of plasma p-tau, p-tau181, p-tau231 and p-tau217 have become subjects of active research in recent years. Several studies have shown that the plasma p-tau181 level gradually increased with the disease progression, and a familial AD exhibited that p-tau181 level showed value in early diagnosis [58-59]. A cross-sectional study compared plasma p-tau231, p-tau217, p-tau181, Aβ42/40, GAFP, and NfL, only p-tau217 change was significantly Aβ protein-dependent over a period of 4-6 years that encompassed both preclinical and symptomatic period, and the p-tau217 level was found to be associated with disease progression, including cognitive decline and brain atrophy [60]. Another study showed that plasma p-tau217 could accurately predict AD within 4 years [61]. It was found that plasma NfL and GFAP could be used to discriminate between patients with AD and healthy individuals, and the increased level of GFAP appeared many years before the pathological state of AD, but since NfL and GFAP are not AD-specific biomarkers, these biomarkers might be used more for diagnosis of neurodegenerative diseases [62-63].
microRNAs (miRNAs) are short non-coding RNAs consisting of 20-25 nucleic acid sequences that bind to mRNAs to prevent translation and thus regulate gene expression post-transcriptionally. The levels of mRNAs related to Aβ production and Tau phosphorylation and their corresponding miRNAs show abnormal changes during the pathological process of AD. miRNAs involved in the pathogenesis of AD enter the bloodstream upon being encapsulated by exosomes or binding to proteins, and their stability is higher than mRNA, so miRNAs transported by exosomes in the blood have attracted attention as potential blood biomarkers. Currently, miRNA-adapted quantitative reverse-transcription polymerase chain reaction (RT-qPCR), microarray hybridization, NanoString sequencing, and next-generation sequencing (NGS) are employed to screen for differences in miRNA expression. Differences in miRNA levels between AD patients and healthy individuals continue to be identified, and further validation is needed to determine which miRNA is more likely to be developed as a blood biomarker for AD. Jia et al. used six neurogenic exosomal miRNAs in plasma to develop a predictive model that could detect AD risk 5-7 years before the onset of AD symptoms[64].
Patients with AD are often already in the pathological stage when they are diagnosed in hospital, and are curable by currently available treatments, and the survival of the patients is often less than 10 years. The biggest advantage of plasma biomarkers lies in that plasma is easy to sample and allows for large-scale screening, which is important for the early diagnosis of AD.
3.4 Emerging nanotechnologically-based detection methods
Although Simoa and MSD enabled the quantification of biomarkers in blood for Alzheimer’s patients, high cost and reliance on specialized instruments limited their more widespread application. Therefore, a cheaper and more convenient detection method is also a research hotspot. The current development of new analytical methods for blood biomarkers of AD is principally based on optoelectronic sensing platforms.
Surface plasmon resonance (SPR) is a phenomenon in which incident light incident at a critical angle to the interface of two media with different refractive indices (such as gold or silver coating on the surface of glass), the resonance of the metal-free electrons can be induced, and due to the resonance of the electrons absorbing the energy of the light, resonance spectra were produced. A common SPR biosensor consists of an optical section, a metal layer, and a sensing layer, where the optical section can be a prism, a fiber optic, or a grating. SPR biosensor is known for quick and easy detection, high sensitivity, and being labeling-free for analyte [65]. If metal nanoparticles are used to replace the metal film layer, the surface plasma wave can be confined to the surface of the nanoparticles, and this kind of resonance phenomenon between the external incident photon and the localized surface plasma wave is called localized surface plasmon resonance (LSPR). Song et al. prepared a plasma biosensor based on DNA-assembled advanced plasma structure (DAPA) for miRNA detection in serum. Two narrow nanogaps in the nanostructure induced plasma coupling between three spherical gold nanoparticles to increase the optical energy density. With a 1.66-fold higher refractive index sensitivity than commonly used gold nanorods at LSPR, the biosensor can even distinguish single nucleotide differences between different miRNAs. Using the biosensor can identify AD patients and healthy controls by detecting the levels of miRNA-125b, miRNA-15a, and miRNA-361 in serum. The sensor showed great potential for clinical diagnosis of AD using miRNAs [66]. Further, Song et al. found that controlling the sodium chloride concentration during the synthesis process could regulate the bending angle of the synthesized gold nanostructures, based on which the researchers synthesized gold nanotriplet spherical structures with 180°, 130°, and 70° angles, and the plasmonic nanostructures with a 130° angle showed higher sensitivity. The biosensor developed based on 130° angle triplet gold nanoparticles for the detection of miRNAs in serum samples allows for the differentiation between healthy individuals, MCI patients, and AD patients. Combining the results of multiple miRNAs, the average diagnostic accuracy reached 98.22% [67].
Surface-enhanced Raman scattering (SERS) is also an option based on LSPR in the detection of Alzheimer’s biomarkers. Raman spectroscopy, which provides vibrational spectra of fingerprint peaks with specific features, is limited as a quantitative detection since its sensitivity is low. SERS was discovered in 1977, and the mechanism has not been fully clarified but is usually attributed to both electromagnetic (EM) and chemical enhancement (CM), with electromagnetic enhancement playing a major role. Raman signaling molecules modified on noble metal surfaces increase the Raman signal by several orders of magnitude, effectively lowering the detection limit. SERS biosensors are mainly composed of plasmonic materials, Raman signaling molecules, and an optional specific capture probe [68]. Zhang et al. developed a colorimetric and SERS dual-mode magnetic immunosensor for the detection of p-tau396,404. The researchers modified p-tau396,404-specific antibodies on superparamagnetic nanoparticles to capture p-tau396,404 in the blood, and gold nanoparticles labeled with Raman signaling molecules are used to produce Roman signals. This dual-mode immunosensor achieved a detection limit of 1.5 pg/mL in SERS mode [69]. Furthermore, on the basis of lateral flow analysis (LFA), researchers developed a colorimetric and SERS dual-mode LFA with a detection limit of 3.8 pg/mL in SERS mode [70].
Detection by electroanalysis is based on changes in electrical properties induced by the analyte, and specific methods include differential pulse voltammetry and electrochemical impedance spectroscopy [71]. Wang et al. developed an electrochemical sensor for β-amyloid oligomers via metal-organic skeleton loaded gold nanoparticles (AuNPs@CuMOF), with a linear range of 0.5 ~ 500 fM and a detection limit of 0.25 fM [72]. Pereira et al. constructed an electrochemical biosensor for miRNA detection based on two gold nanostructure-modified carbon screen-printed electrodes (C-SPEs) and an oligonucleotide probe for miR-34a, which showed a linear range of 100 pM - 1 μM, with a detection limit of 94 aM in serum [73].
4 CONCLUSION AND FUTURE DIRECTION
This review falls into two parts. In the first part, we review the development of diagnostic criteria for AD formulated by NIA-AA, and the term “biomarker” appeared, for the first time, in the diagnostic criteria issued in 2011. A biological definition of AD was given by the clinical research framework published in 2018, while AD was defined by clinical symptoms in the past decade. In 2023, the latest draft of the revised diagnostic criteria improves the categorization of biomarkers and delineates the function of different biomarkers based on current research. It’s reasonable to believe that biomarkers will be increasingly used in the diagnosis of AD, because biomarkers provide more objective results, and abnormal changes in biomarkers precede the appearance of clinical symptoms. In using biomarkers as a main diagnostic tool for AD, the following issues are important: (1) As AD progresses continuously, the determination of thresholds for diagnosis and staging of AD by biomarkers is critical. This requires more clinical data and experience. (3) After the biomarker diagnostic thresholds are determined, how to deal with the value near the thresholds. Artificial Intelligence can give doctors advice by learning on constant basis [74].
In the second part, we discussed the detection methods for AD biomarkers. Both detection of biomarkers for AD in CSF and imaging already are included in FDA-approved assays [75-76]. Although not for all biomarkers of AD, it’s still essential for the further application of biomarkers in Alzheimer’s diagnosis. In contrast, no detection methods for plasma biomarkers have received regulatory approval, and the main problems with plasma biomarkers include: (1) Ideal AD plasma biomarker levels should evolve with AD progression, and current reports indicated that p-tau217 is advantageous over other biomarkers [60], and further validation is needed to determine the ultimate application of plasma biomarkers, in a combined multi-marker or single-marker fashion. (2) Currently, no study showed which plasma biomarker assay is equivalent to cerebrospinal fluid biomarkers and PET assays in terms of performance. (3) The same biomarker showed a much lower level in plasma than in CSF, which means absolute quantification is difficult, and the cost of high-sensitivity assay is much higher than traditional methods such as ELISA. Substantial discrepancies existed between the results yielded by different testing methods [77-78]. All these factors restrict the further development of plasma biomarkers. The diagnosis of AD solely through blood collection is an ideal model, and researchers in the nanotechnology field have been endeavoring towards this goal.
In recent years, the development of assays for AD diagnosis in the nano field has been focusing on SPR and electrochemical methods, which are also commonly used for the detection of blood biomarkers in cancer and other diseases. All the efforts are directed at developing convenient, sensitive, and cheaper assays. Except for the protein biomarkers, miRNA was also under test, which broadens the range of biomarkers that can be tested for the diagnosis of AD. Most of the existing nanotechnology-based diagnostic tools require tightly controlled experimental conditions, which is unfriendly to clinical applications [79-80]. The nanotechnology-based diagnostic tools for AD could create more possibilities for their industrial production and application if they can achieve a high level of reproducibility and stability.
ACKNOWLEDGMENTS
This work was financially supported by the National Natural Science Foundation of China (82374175, 82304915).
COMPETING INTERESTS
The authors have declared that no competing interests exist.
ABBREVIATION USED
AD, Alzheimer’s disease; CSF, cerebrospinal fluid; PET, positron emission tomography; ADRDA, the Alzheimer’s Diease and Related Disorders Association; NINCDS, the National Institute of Neurological and Communicative Disorders and Stroke; NIA-AA, the National Institute on Aging and Alzheimer’s Association ; ELISA, enzyme linked immunosorbent assay; Simoa, single-molecule Array; CT, computerized tomography; MRI, magnetic resonance imaging; AD-P, process of Alzheimer’s disease; AD-C, clinical syndrome of Alzheimer’s disease; MCI, Mild cognitive impairment; Aβ, amyloid β; FDG, fludeoxyglucose; SPECT, Single-Photon Emission Computed Tomography; APP, Amyloid precursor protein; PHFs, Paired helical filaments; NFTs, Neurofibrillary tangles; CCD, Charge-Coupled Device; LOD, Limit of detection; LOQ, Limit of quantitation; miRNAs, microRNAs; RT-qPCR, Reverse-Transcription Polymerase Chain Reaction; NGS, Next-Generation Sequencing; SPR, Surface plasmon resonance; DAPA, DNA-assembled advanced plasma structure; SERS, Surface-enhanced Raman scattering; EM, electromagnetic; CM, chemical enhancement; LFA, lateral flow analysis; C-SPEs, Carbon Screen-Printed Electrodes; MSD, Meso Scale Discovery; TPA, Tripropylamine; LSPR, localized surface plasmon resonance: CNS, central nervous system.
REFERENCES
- 1.2022 Alzheimer's disease facts and figures. Alzheimers Dement Apr;18(4):700-789. 10.1002/alz.12638 PMID: . [DOI] [PubMed] [Google Scholar]
- 2.2023 Alzheimer's disease facts and figures. Alzheimers Dement Apr;19(4):1598-1695. 10.1002/alz.13016 PMID: . [DOI] [PubMed] [Google Scholar]
- 3.Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E:high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993. Mar;90(5):1977-81. 10.1073/pnas.90.5.1977 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayeux R, Saunders AM, Shea S, Mirra S, Evans D, et al. Utility of the apolipoprotein E genotype in the diagnosis of Alzheimer's disease. Alzheimer's Disease Centers Consortium on Apolipoprotein E and Alzheimer's Disease. N Engl J Med. 1998. Feb;338(8):506-11. 10.1056/NEJM199802193380804 PMID: . [DOI] [PubMed] [Google Scholar]
- 5.Zhong G, Wang Y, Zhang Y, Guo JJ, Zhao Y. Smoking is associated with an increased risk of dementia:a meta-analysis of prospective cohort studies with investigation of potential effect modifiers. PLoS One. 2015. Mar;10(3):e0118333. 10.1371/journal.pone.0118333 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butler M, Nelson VA, Davila H, Ratner E, Fink HA, Hemmy LS, McCarten JR, Barclay TR, Brasure M, Kane RL. Over-the-Counter Supplement Interventions to Prevent Cognitive Decline, Mild Cognitive Impairment, and Clinical Alzheimer-Type Dementia:A Systematic Review. Ann Intern Med. 2018. Jan;168(1):52-62. 10.7326/M17-1530 PMID: . [DOI] [PubMed] [Google Scholar]
- 7.Morris MC, Tangney CC, Wang Y, Sacks FM, Barnes LL, Bennett DA, Aggarwal NT. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015. Sep;11(9):1015-22. 10.1016/j.jalz.2015.04.011 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chetelat G, Teunissen CE, et al. Alzheimer's disease. Lancet. 2021. Apr 24;397(10284):1577-1590. 10.1016/S0140-6736(20)32205-4 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease:report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984. Jul;34(7):939-44. 10.1212/wnl.34.7.939 PMID: . [DOI] [PubMed] [Google Scholar]
- 10.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease:recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011. May;7(3):270-9. 10.1016/jjalz.2011.03.008 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jack CR, Jr, Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011. May;7(3):257-62. 10.1016/jjalz.2011.03.004 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease:recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011. May;7(3):263-9. 10.1016/jjalz.2011.03.005 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease:recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011. May;7(3):280-92. 10.1016/jjalz.2011.03.003 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jack CR, Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework:Toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018. Apr;14(4):535-562. 10.1016/jjalz.2018.02.018 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975. Nov;12(3):189-98. 10.1016/0022-3956(75)90026-6 PMID: . [DOI] [PubMed] [Google Scholar]
- 16.Hachinski VC, Iliff LD, Zilhka E, Du Boulay GH, McAllister VL, Marshall J, Russell RW, Symon L. Cerebral blood flow in dementia. Arch Neurol. 1975. Sep;32(9):632-7. 10.1001/archneur.1975.00490510088009 PMID: . [DOI] [PubMed] [Google Scholar]
- 17.Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, Bi W, Cohen AD, Lopresti BJ, DeKosky ST, Halligan EM, Klunk WE. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008. Nov;65(11):1509-17. 10.1001/archneur.65.11.1509 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jack CR, Jr, Therneau TM, Weigand SD, Wiste HJ, Knopman DS, et al. Prevalence of Biologically vs Clinically Defined Alzheimer Spectrum Entities Using the National Institute on Aging-Alzheimer's Association Research Framework. JAMANeurol. 2019. Jul;76(10):1174-83. 10.1001/jamaneurol.2019.1971 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louie R. The 2018 NIA-AA research framework:Recommendation and comments. Alzheimers Dement. 2019. Jan;15(1):182-183. 10.1016/j.jalz.2018.06.3062 PMID: . [DOI] [PubMed] [Google Scholar]
- 20.McRae-McKee K, Udeh-Momoh CT, Price G, Bajaj S, de Jager CA, et al. Perspective:Clinical relevance of the dichotomous classification of Alzheimer's disease biomarkers:Should there be a “gray zone”?. Alzheimers Dement. 2019. Oct;15(10):1348-1356. 10.1016/jjalz.2019.07.010 PMID: . [DOI] [PubMed] [Google Scholar]
- 21.Tang Y, Lutz MW, Xing Y. A systems-based model of Alzheimer's disease. Alzheimers Dement. 2019. Jan;15(1):168-171. 10.1016/j.jalz.2018.06.3058 PMID: . [DOI] [PubMed] [Google Scholar]
- 22.Aguillon D, Langella S, Chen Y, Sanchez JS, Su Y, Vila-Castelar C, et al. Plasma p-tau217 predicts in vivo brain pathology and cognition in autosomal dominant Alzheimer's disease. Alzheimers Dement. 2023. Jun;19(6):2585-2594. 10.1002/alz.12906 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ossenkoppele R, van der Kant R, Hansson O. Tau biomarkers in Alzheimer's disease:towards implementation in clinical practice and trials. Lancet Neurol. 2022. Aug;21(8):726-734. 10.1016/S1474-4422(22)00168-5 PMID: . [DOI] [PubMed] [Google Scholar]
- 24.Alzheimer's Association International Conference [Internet]. Alzheimer's Association. [cited 2023 Dec 9]. Available from: https://aaic.alz.org/diagnostic-criteria.asp.
- 25.Alzheimer's Association International Conference [Internet]. Alzheimer's Association. [cited 2023 Dec 9]. Available from: https://alz.org/media/Documents/scientific-conferences/Clinical-Criteria-for-Staging-and-Diagnosis-for-Public-Comment-Draft-2.pdf?_gl=1*lgta1s*_ga*MTc2NjMxMjA1Ni4xNzAxNzU5MDg5*_ga_9JTEWVX24V*MTcwMjM1MTQ4MS45LjEuMTcwMjM1MT cyNy42MC4wLjA.*_ga_QSFTKCEH7C*MTcwMjM1MTQ4MS 45LjEuMTcwMjM1MTcyNy42MC4wLjA.
- 26.Alzheimer's Association International Conference [Internet]. Alzheimer's Association. [cited 2023 Dec 9]. Available from: https://alz.org/media/Documents/scientific-conferences/ Figures-and-Tables-Clinical-Criteria-for-Staging-and-Diagnosis-for-Public-Comment-Draft-2.pdf?_gl=1*1depbc6*_ga *MTc 2Nj MxMj A1Ni4xNzAxNzU5MDg5*_ga_9JTEWVX24V*MTcwMjM2MDI2Mi4xMC4wLjE3MDIzNj AyNjIuNjAuMC4w*_ga_QSFTKCEH7C*MTcwMjM2MDI2Mi4 xMC4wLjE3MDIzNjAyNjIuNjAuMC4w.
- 27.Barichello T, Giridharan VV, Dal-Pizzol F. A cerebrospinal fluid biosignature for the diagnosis of Alzheimer's disease. Braz J Psychiatry. 2019. Nov-Dec;41(6):467-468. 10.1590/1516-4446-2019-0629 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vermunt L, Sikkes SAM, van den Hout A, Handels R, Bos I, van der Flier WM, et al. Duration of preclinical, prodromal, and dementia stages of Alzheimer's disease in relation to age, sex, and APOE genotype. Alzheimers Dement. 2019. Jul;15(7):888-898. 10.1016/j.jalz.2019.04.001 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Self WK, Holtzman DM. Emerging diagnostics and therapeutics for Alzheimer disease. Nat Med. 2023. Sep;29(9):2187-2199. 10.1038/s41591-023-02505-2 PMID: . [DOI] [PubMed] [Google Scholar]
- 30.Johnson ECB, Bian S, Haque RU, Carter EK, Watson CM, Gordon BA, et al. Cerebrospinal fluid proteomics define the natural history of autosomal dominant Alzheimer's disease. Nat Med. 2023. Aug;29(8):1979-1988. 10.1038/s41591-023-02476-4 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hansson O, Lehmann S, Otto M, Zetterberg H, Lewczuk P. Advantages and disadvantages of the use of the CSF Amyloid p (Ap) 42/40 ratio in the diagnosis of Alzheimer's Disease. Alzheimers Res Ther. 2019. Apr 22;11(1):34. 10.1186/s13195-019-0485-0 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mattsson N. CSF biomarkers in neurodegenerative diseases. Clin Chem Lab Med. 2011. Mar;49(3):345-52. 10.1515/CCLM.2011.082 PMID: . [DOI] [PubMed] [Google Scholar]
- 33.Ost M, Nylen K, Csajbok L, Ohrfelt AO, Tullberg M, Wikkelso C, et al. Initial CSF total tau correlates with 1-year outcome in patients with traumatic brain injury. Neurology. 2006. Nov 14;67(9):1600-4. 10.1212/01.wnl.0000242732.06714.0f PMID: . [DOI] [PubMed] [Google Scholar]
- 34.Olsson B, Lautner R, Andreasson U, Ohrfelt A, Portelius E, Bjerke M, et al. CSF and blood biomarkers for the diagnosis of Alzheimer's disease:a systematic review and meta-analysis. Lancet Neurol. 2016. Jun;15(7):673-684. 10.1016/S1474-4422(16)00070-3 PMID: . [DOI] [PubMed] [Google Scholar]
- 35.Skillback T, Rosen C, Asztely F, Mattsson N, Blennow K, Zetterberg H. Diagnostic performance of cerebrospinal fluid total tau and phosphorylated tau in Creutzfeldt-Jakob disease:results from the Swedish Mortality Registry. JAMA Neurol. 2014. Apr;71(4):476-83. 10.1001/jamaneurol.2013.6455 PMID: . [DOI] [PubMed] [Google Scholar]
- 36.Suarez-Calvet M, Karikari TK, Ashton NJ, Lantero Rodriguez J, Mila-Aloma M, Gispert JD, et al. Novel tau biomarkers phosphorylated at T181, T217 or T231 rise in the initial stages of the preclinical Alzheimer's continuum when only subtle changes in Ap pathology are detected. EMBO Mol Med. 2020. Dec 7;12(12):e12921. 10.15252/emmm.202012921 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaw LM, Arias J, Blennow K, Galasko D, Molinuevo JL, Salloway S, et al. Appropriate use criteria for lumbar puncture and cerebrospinal fluid testing in the diagnosis of Alzheimer's disease. Alzheimers Dement. 2018. Nov;14(11):1505-1521. 10.1016/j.jalz.2018.07.220 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Meyer S, Schaeverbeke JM, Verberk IMW, Gille B, De Schaepdryver M, Luckett ES, et al. Comparison of ELISA- and SIMOA-based quantification of plasma Ap ratios for early detection of cerebral amyloidosis. Alzheimers Res Ther. 2020. Dec 5;12(1):162. 10.1186/s13195-020-00728-w PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuhle J, Barro C, Andreasson U, Derfuss T, Lindberg R, Sandelius A, et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples:ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med. 2016. Oct 1;54(10):1655-61. 10.1515/cclm-2015-1195 PMID: . [DOI] [PubMed] [Google Scholar]
- 40.Burkett BJ, Babcock JC, Lowe VJ, Graff-Radford J, Subramaniam RM, Johnson DR. PET Imaging of Dementia:Update 2022. Clin Nucl Med. 2022. Sep;47(9):763-773. 10.1097/RLU.0000000000004251 PMID: . [DOI] [PubMed] [Google Scholar]
- 41.Villemagne VL. Amyloid imaging:Past, present and future perspectives. Ageing Res Rev. 2016. Sep;30:95-106. 10.1016/j.arr.2016.01.005 PMID: . [DOI] [PubMed] [Google Scholar]
- 42.Morris E, Chalkidou A, Hammers A, Peacock J, Summers J, Keevil S. Diagnostic accuracy of (18)F amyloid PET tracers for the diagnosis of Alzheimer's disease:a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2016. Feb;43(2):374-385. 10.1007/s00259-015-3228-x PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997. Jul-Aug;18(4):351-7. 10.1016/s0197-4580(97)00056-0 PMID: . [DOI] [PubMed] [Google Scholar]
- 44.Chien DT, Bahri S, Szardenings AK, Walsh JC, Mu F, Su MY, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J Alzheimers Dis. 2013;34(2):457-68. 10.3233/JAD-122059 PMID: . [DOI] [PubMed] [Google Scholar]
- 45.Lowe VJ, Curran G, Fang P, Liesinger AM, Josephs KA, Parisi JE, et al. An autoradiographic evaluation of AV-1451 Tau PET in dementia. Acta Neuropathol Commun. 2016. Jun 13;4(1):58. 10.1186/s40478-016-0315-6 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwarz AJ, Yu P, Miller BB, Shcherbinin S, Dickson J, Navitsky M, et al. Regional profiles of the candidate tau PET ligand 18F-AV-1451 recapitulate key features of Braak histopathological stages. Brain. 2016. May;139(Pt 5):1539-50. 10.1093/brain/aww023 PMID: . [DOI] [PubMed] [Google Scholar]
- 47.Tian M, Civelek AC, Carrio I, Watanabe Y, Kang KW, Murakami K, et al. International consensus on the use of tau PET imaging agent 18F-flortaucipir in Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2022. Feb;49(3):895-904. 10.1007/s00259-021-05673-w PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teunissen CE, Verberk IMW, Thijssen EH, Vermunt L, Hansson O, Zetterberg H, et al. Blood-based biomarkers for Alzheimer's disease:towards clinical implementation. Lancet Neurol. 2022. Jan;21(1):66-77. 10.1016/S1474-4422(21)00361-6 PMID: . [DOI] [PubMed] [Google Scholar]
- 49.Hampel H, Hu Y, Cummings J, Mattke S, Iwatsubo T, Nakamura A, et al. Blood-based biomarkers for Alzheimer's disease:Current state and future use in a transformed global healthcare landscape. Neuron. 2023. Sep;111(18):2781-2799. 10.1016/j.neuron.2023.05.017 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pais MV, Forlenza OV, Diniz BS. Plasma Biomarkers ofAlzheimer's Disease:A Review of Available Assays, Recent Developments, and Implications for Clinical Practice. J Alzheimers Dis Rep. 2023. May;7(1):355-380. 10.3233/ADR-230029 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson DH, Rissin DM, Kan CW, Fournier DR, Piech T, Campbell TG, et al. The Simoa HD-1 Analyzer:A Novel Fully Automated Digital Immunoassay Analyzer with Single-Molecule Sensitivity and Multiplexing. J Lab Autom. 2016. Aug;21(4):533-47. 10.1177/2211068215589580 PMID: . [DOI] [PubMed] [Google Scholar]
- 52.Li D, Mielke MM. An Update on Blood-Based Markers of Alzheimer's Disease Using the Simoa Platform. Neurol Ther. 2019. Dec;8(Suppl 2):73-82. 10.1007/s40120-019-00164-5 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ding XL, Tuo QZ, Lei P. An Introduction to Ultrasensitive Assays for Plasma Tau Detection. J Alzheimers Dis. 2021;80(4):1353-1362. 10.3233/JAD-201499 PMID: . [DOI] [PubMed] [Google Scholar]
- 54.Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Dore V, et al. High performance plasma amyloid-P biomarkers for Alzheimer's disease. Nature. 2018. Feb 8;554(7691):249-254. 10.1038/nature25456 PMID: . [DOI] [PubMed] [Google Scholar]
- 55.Keshavan A, Pannee J, Karikari TK, Rodriguez JL, Ashton NJ, Nicholas JM, et al. Population-based blood screening for preclinical Alzheimer's disease in a British birth cohort at age 70. Brain. 2021. Mar 3;144(2):434-449. 10.1093/brain/awaa403 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schindler SE, Bollinger JG, Ovod V, Mawuenyega KG, Li Y, Gordon BA, et al. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology. 2019. Oct 22;93(17):e1647-e1659. 10.1212/WNL.0000000000008081 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ovod V, Ramsey KN, Mawuenyega KG, Bollinger JG, Hicks T, Schneider T, et al. Amyloid P concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement. 2017. Aug;13(8):841-849. 10.1016/j.jalz.2017.06.2266 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thijssen EH, La Joie R, Wolf A, Strom A, Wang P, Iaccarino L, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer's disease and frontotemporal lobar degeneration. Nat Med. 2020. Mar;26(3):387-397. 10.1038/s41591-020-0762-2 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lantero Rodriguez J, Karikari TK, Suarez-Calvet M, Troakes C, King A, Emersic A, et al. Plasma p-tau181 accurately predicts Alzheimer's disease pathology at least 8 years prior to post-mortem and improves the clinical characterisation of cognitive decline. Acta Neuropathol. 2020. Sep;140(3):267-278. 10.1007/s00401-020-02195-x PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ashton NJ, Janelidze S, Mattsson-Carlgren N, Binette AP, Strandberg O, Brum WS, et al. Differential roles of Aβ42/40, p-tau231 and p-tau217 for Alzheimer's trial selection and disease monitoring. Nat Med. 2022. Dec;28(12):2555-2562. 10.1038/s41591-022-02074-w PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palmqvist S, Tideman P, Cullen N, Zetterberg H, Blennow K, et al. Prediction of future Alzheimer's disease dementia using plasma phospho-tau combined with other accessible measures. Nat Med Jun;27(6):1034-1042. 10.1038/s41591-021-01348-z PMID: . [DOI] [PubMed] [Google Scholar]
- 62.Stocker H, Beyer L, Perna L, Rujescu D, Holleczek B, et al. Association of plasma biomarkers, p-tau181, glial fibrillary acidic protein, and neurofilament light, with intermediate and long-term clinical Alzheimer's disease risk:Results from a prospective cohort followed over 17 years. Alzheimers Dement. 2023. Jan;19(1):25-35. 10.1002/alz.12614 PMID: . [DOI] [PubMed] [Google Scholar]
- 63.Johansson C, Thordardottir S, Laffita-Mesa J, Rodriguez-Vieitez E, Zetterberg H, et al. Plasma biomarker profiles in autosomal dominant Alzheimer's disease. Brain. 2023. Mar 1;146(3):1132-1140. 10.1093/brain/awac399 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jia L, Zhu M, Yang J, Pang Y, Wang Q, et al. Exosomal MicroRNA-Based Predictive Model for Preclinical Alzheimer's Disease:A Multicenter Study. Biol Psychiatry. 2022. Jul 1;92(1):44-53. 10.1016/j.biopsych.2021.12.015 PMID: . [DOI] [PubMed] [Google Scholar]
- 65.Wang Q, Ren ZH, Zhao WM, Wang L, Yan X, et al. Research advances on surface plasmon resonance biosensors. Nanoscale Jan 20;14(3):564-591. 10.1039/d1nr05400g PMID: . [DOI] [PubMed] [Google Scholar]
- 66.Song S, Lee JU, Jeon MJ, Kim S, Sim SJ. Detection of multiplex exosomal miRNAs for clinically accurate diagnosis ofAlzheimer's disease using label-free plasmonic biosensor based on DNA-Assembled advanced plasmonic architecture. Biosens Bioelectron. 2022. Mar 1;199:113864. 10.1016/j.bios.2021.113864 PMID: . [DOI] [PubMed] [Google Scholar]
- 67.Song S, Lee JU, Jeon MJ, Kim S, Lee CN, Sim SJ. Precise profiling of exosomal biomarkers via programmable curved plasmonic nanoarchitecture-based biosensor for clinical diagnosis of Alzheimer's disease. Biosens Bioelectron. 2023. Jun 15;230:115269. 10.1016/j.bios.2023.115269 PMID: . [DOI] [PubMed] [Google Scholar]
- 68.Li Q, Huo H, Wu Y, Chen L, Su L, et al. Design and Synthesis of SERS Materials for In Vivo Molecular Imaging and Biosensing. Adv Sci (Weinh). 2023. Mar;10(8):e2202051. 10.1002/advs.202202051 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang L, Cao K, Su Y, Hu S, Liang X, et al. Colorimetric and surface-enhanced Raman scattering dual-mode magnetic immunosensor for ultrasensitive detection of blood phosphorylated tau in Alzheimer's disease. Biosens Bioelectron. 2023. Feb 15;222(114935): 10.1016/j.bios.2022.114935 PMID: . [DOI] [PubMed] [Google Scholar]
- 70.Zhang L, Su Y, Liang X, Cao K, Luo Q, Luo H. Ultrasensitive and point-of-care detection of plasma phosphorylated tau in Alzheimer's disease using colorimetric and surface-enhanced Raman scattering dual-readout lateral flow assay. Nano Res. 2023;16(5):7459-7469. 10.1007/s12274-022-5354-4 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hu S, Yang C, Luo H. Current trends in blood biomarker detection and imaging for Alzheimer's disease. Biosens Bioelectron. 2022. Aug 15;210:114278. 10.1016/j.bios.2022.114278 PMID: . [DOI] [PubMed] [Google Scholar]
- 72.Wang X, Li L, Gu X, Yu B, Jiang M. Switchable electrochemical aptasensor for amyloid-P oligomers detection based on triple helix switch coupling with AuNPs@CuMOF labeled signaling displaced-probe. Mikrochim Acta. 2021. Jan 25;188(2):49. 10.1007/s00604-021-04704-5 PMID: . [DOI] [PubMed] [Google Scholar]
- 73.Pereira RL, Oliveira D, Pego AP, Santos SD, Moreira FTC. Electrochemical miRNA-34a-based biosensor for the diagnosis ofAlzheimer's disease. Bioelectrochemistry. 2023. Dec;154:108553. 10.1016/j.bioelechem.2023.108553 PMID: . [DOI] [PubMed] [Google Scholar]
- 74.Sun J, Dong QX, Wang SW, Zheng YB, Liu XX, Lu TS, et al. Artificial intelligence in psychiatry research, diagnosis, and therapy. Asian J Psychiatr. 2023. Sep;87:103705. 10.1016/j.ajp.2023.103705 PMID: . [DOI] [PubMed] [Google Scholar]
- 75.FDA Permits Marketing for New Test to Improve Diagnosis of Alzheimer's Disease [Internet]. U. S. Food &Drug administration. [cited 2023 Dec 9]. Available from: https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-new-test-improve-diagnosis-alzheimers-disease..
- 76.FDA Permits Marketing for New Test to Improve Diagnosis of Alzheimer's Disease [Internet]. U. S. Food &Drug administration. [cited 2023 Dec 9]. https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-image-tau-pathology-patients-being-evaluated-alzheimers-disease.
- 77.Janelidze S, Bali D, Ashton NJ, Barthelemy NR, Vanbrabant J, et al. Head-to-head comparison of 10 plasma phospho-tau assays in prodromal Alzheimer's disease. Brain. 2023. Apr;146(4):1592-1601. 10.1093/brain/awac333. PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ashton NJ, Puig-Pijoan A, Mila-Aloma M, Fernandez-Lebrero A, Garda-Escobar G, et al. Plasma and CSF biomarkers in a memory clinic:Head-to-head comparison of phosphorylated tau immunoassays. Alzheimers Dement. 2023. May;19(5):1913-1924. 10.1002/alz.12841 PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang X, Huang SC, Hu S, et al. Fundamental understanding and applications of plasmon-enhanced Raman spectroscopy. Nat Rev Phys. 2020. May;2:253-271. 10.1038/s42254-020-0171-y. [DOI] [Google Scholar]
- 80.Han X. X, Rodriguez R. S, Haynes C. L, et al. Surface-enhanced Raman spectroscopy. Nat Rev Methods Primers. 2022. Jan;1:87. 10.1038/s43586-021-00083-6. [DOI] [Google Scholar]